
In inorganic chemistry, silenes, or disilalkenes, [1] are silicon compounds that contain Si=Si double bonds, where the oxidation state of Si is +2. The parent molecule is disilene, Si2H4.

In inorganic chemistry, silenes, or disilalkenes, [1] are silicon compounds that contain Si=Si double bonds, where the oxidation state of Si is +2. The parent molecule is disilene, Si2H4.
The first transient disilene was reported in 1972 by D. N. Roark and Garry J. D. Peddle. Simple disilenes easily polymerize. To suppress this tendency, bulky substituents are used. Indeed the first isolable disilene, tetramesityldisilene, was described in 1981 by West, Fink, and Michl. [2] [3] It was prepared by UV-photolysis of the related cyclic trisilane:
Tetramesityldisilene (CH3)2Si=Si(CH3)2 is a yellow-orange solid. The Si=Si double bond lengths of disilenes vary between 2.14 and 2.29 Å and are nearly 5 to 10% shorter than the Si-Si single bond lengths of corresponding disilanes. A peculiarity of disilenes is the trans-bending of the substituents, which is never observed in alkenes. The trans-bent angles of disilenes between the R2Si planes and the Si=Si vector range from 0 to 33.8 °. This distortion is rationalized by the stability of the corresponding silylene fragments, although disilenes do not typically dissociate.
The distorted geometry of disilenes can be rationalized by considering the valence orbitals of silicon, which are 3s and 3p, whereas those of carbon are 2s and 2p. Thus, the energy gap between the ns and np orbitals of a silicon atom is larger than that of a carbon atom. Therefore, silylene fragments are in a singlet state, while carbene fragments are in a triplet state. So, when double bonds are formed by the interaction of these two fragments, disilenes which consist of two silylene units are trans-bending and alkenes which consist of two carbene units are planar. The bending is even more extreme for the tin analogues of disilenes. [1]
Disilenes are generally synthesized by reduction of 1,2-dihalodisilane, by retro-Diels–Alder fragmentation, by dimerization of silylenes, by photofragmentation of cyclopolysilanes, or by rearrangement of silylsilylenes.
A series of 1,1,1,4,4,4-hexaalkyl-2,3-bis(trialkylsilyl)tetrasil-2-enes structural analogues are typically synthesised using reductive coupling of the corresponding 1,1,1,3,3,3-hexaalkyl-2,3-tribromotrisilanes.
To form the root of the IUPAC names for silenes, simply change the -an- infix of the parent to -en-. For example, SiH
3-SiH
3 is the silane disilANe. The name of SiH
2=SiH
2 is therefore disilENe.
In higher silenes, where isomers exist that differ in location of the double bond, the following numbering system is used:

In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".
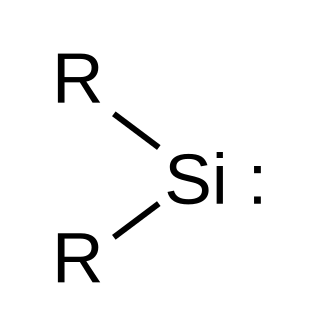
Silylene is a chemical compound with the formula SiR2. It is the silicon analog of carbene. Due to presence of a vacant p orbital, silylene rapidly reacts in a bimolecular manner when condensed. Unlike carbenes, which can exist in the singlet or triplet state, silylene (and all of its derivatives) are singlets.
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane and used in many chemical processes. Each such chemical has at least one silicon-chlorine bond. Trichlorosilane is produced on the largest scale. The parent chlorosilane is silicon tetrachloride.

Organosilicon chemistry is the study of organometallic compounds containing carbon–silicon bonds, to which they are called organosilicon compounds. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an inorganic compound.
A silylenoid in organosilicon chemistry is a type of chemical compound with the general structure R2SiXM where R is any organic residue, X a halogen and M a metal. Silylenoids are the silicon pendants of carbenoid and both compounds have carbene or silylene like properties.

Silanes are saturated chemical compounds with the empirical formula SixHy. They are hydrosilanes, a class of compounds that includes compounds with Si−H and other Si−X bonds. All contain tetrahedral silicon and terminal hydrides. They only have Si−H and Si−Si single bonds. The bond lengths are 146.0 pm for a Si−H bond and 233 pm for a Si−Si bond. The structures of the silanes are analogues of the alkanes, starting with silane, SiH4, the analogue of methane, continuing with disilane Si2H6, the analogue of ethane, etc. They are mainly of theoretical or academic interest.
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen, chalcogen, or halogen. The oldest-known onium ion, and the namesake for the class, is ammonium, NH+4, the protonated derivative of ammonia, NH3.

Disilyne is a low valent silicon compound with the chemical formula Si2R2 where oxidation state of Si is +1. Several isomers are possible, but none are sufficiently stable to be of practical value. Substituted disilynes contain a formal silicon–silicon triple bond and as such are sometimes written R2Si2 (where R is a substituent group). They are the silicon analogues of alkynes.

Polysilanes are organosilicon compounds with the formula (R2Si)n. They are relatives of traditional organic polymers but their backbones are composed of silicon atoms. They exhibit distinctive optical and electrical properties. They are mainly used as precursors to silicon carbide. The simplest polysilane would be (SiH2)n, which is mainly of theoretical, not practical interest.
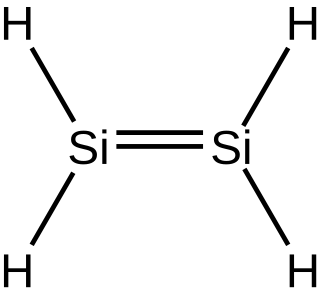
Disilene is an inorganic compound with the chemical formula Si
2H
4. The name disilene, referring to the structure of a particular prototropic tautomer of the molecule. It is the simplest silene.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.
Digermynes are a class of compounds that are regarded as the heavier digermanium analogues of alkynes. The parent member of this entire class is H-Ge≡Ge-H, which has only been characterized computationally, but has revealed key features of the whole class. Because of the large interatomic repulsion between two Ge atoms, only kinetically stabilized digermyne molecules can be synthesized and characterized by utilizing bulky protecting groups and appropriate synthetic methods, for example, reductive coupling of germanium(II) halides.
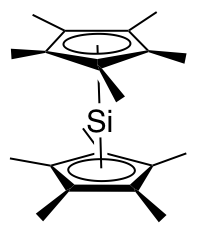
Decamethylsilicocene, (C5Me5)2Si, is a group 14 sandwich compound. It is an example of a main-group cyclopentadienyl complex; these molecules are related to metallocenes but contain p-block elements as the central atom. It is a colorless, air sensitive solid that sublimes under vacuum.

Trisilaallene is a subclass of silenes derivatives where a central silicon atom forms double bonds with each of two terminal silicon atoms, with the generic formula R2Si=Si=SiR2. Trisilaallene is a silicon-based analog of an allene, but their chemical properties are markedly different.

Phosphasilenes or silylidenephosphanes are a class of compounds with silicon-phosphorus double bonds. Since the electronegativity of phosphorus (2.1) is higher than that of silicon (1.9), the "Si=P" moiety of phosphasilene is polarized. The degree of polarization can be tuned by altering the coordination numbers of the Si and P centers, or by modifying the electronic properties of the substituents. The phosphasilene Si=P double bond is highly reactive, yet with the choice of proper substituents, it can be stabilized via donor-acceptor interaction or by steric congestion.

An N-Heterocyclic silylene (NHSi) is a neutral heterocyclic chemical compound consisting of a divalent silicon atom bonded to two nitrogen atoms. The isolation of the first stable NHSi, also the first stable dicoordinate silicon compound, was reported in 1994 by Michael Denk and Robert West three years after Anthony Arduengo first isolated an N-heterocyclic carbene, the lighter congener of NHSis. Since their first isolation, NHSis have been synthesized and studied with both saturated and unsaturated central rings ranging in size from 4 to 6 atoms. The stability of NHSis, especially 6π aromatic unsaturated five-membered examples, make them useful systems to study the structure and reactivity of silylenes and low-valent main group elements in general. Though not used outside of academic settings, complexes containing NHSis are known to be competent catalysts for industrially important reactions. This article focuses on the properties and reactivity of five-membered NHSis.

Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species.
In organosilicon chemistry, silanes are a diverse class of charge-neutral organic compounds with the general formula SiR4. The R substituents can be any combination of organic or inorganic groups. Most silanes contain Si-C bonds, and are discussed under organosilicon compounds. Some contain Si-H bonds and are discussed under hydrosilanes.
In chemistry, transition metal silyl complexes describe coordination complexes in which a transition metal is bonded to an anionic silyl ligand, forming a metal-silicon sigma bond. This class of complexes are numerous and some are technologically significant as intermediates in hydrosilylation. These complexes are a subset of organosilicon compounds.