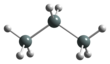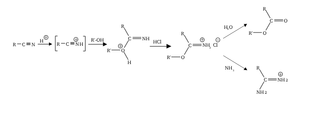
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt ; this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first described it in 1877. Pinner salts are themselves reactive and undergo additional nucleophilic additions to give various useful products:
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colourless, pyrophoric, toxic gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Silanes are commonly used to apply coatings to surfaces or as an adhesion promoter.
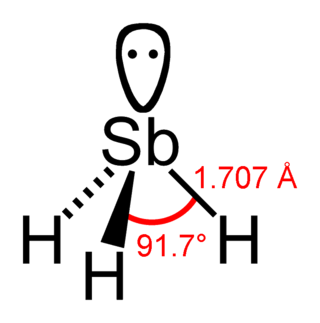
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs).
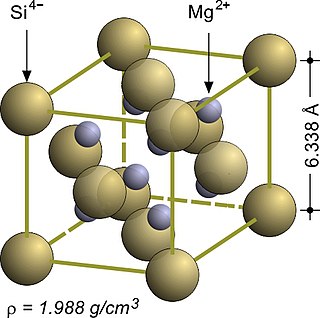
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As-grown Mg2Si usually forms black crystals; they are semiconductors with n-type conductivity and have potential applications in thermoelectric generators.

Indene is an aromatic, polycyclic hydrocarbon with chemical formula C9H8. It is composed of a benzene ring fused with a cyclopentene ring. This flammable liquid is colorless although samples often are pale yellow. The principal industrial use of indene is in the production of indene/coumarone thermoplastic resins. Substituted indenes and their closely related indane derivatives are important structural motifs found in many natural products and biologically active molecules, such as sulindac.

Germane is the chemical compound with the formula GeH4, and the germanium analogue of methane. It is the simplest germanium hydride and one of the most useful compounds of germanium. Like the related compounds silane and methane, germane is tetrahedral. It burns in air to produce GeO2 and water. Germane is a group 14 hydride.

The Reimer–Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols. with the simplest example being the conversion of phenol to salicylaldehyde. The reaction was first reported by Karl Reimer and Ferdinand Tiemann.
Disilane is a chemical compound with chemical formula Si2H6 that was identified in 1902 by Henri Moissan and Samuel Smiles (1877–1953). Moissan and Smiles reported disilane as being among the products formed by the action of dilute acids on metal silicides. Although these reactions had been previously investigated by Friedrich Woehler and Heinrich Buff between 1857 and 1858, Moissan and Smiles were the first to explicitly identify disilane. They referred to disilane as silicoethane. Higher members of the homologous series SinH2n+2 formed in these reactions were subsequently identified by Carl Somiesky and Alfred Stock.

Silanes are saturated chemical compounds with the empirical formula SixHy. They are hydrosilanes, a class of compounds that includes compounds with Si−H and other Si−X bonds. All contain tetrahedral silicon and terminal hydrides. They only have Si−H and Si−Si single bonds. The bond lengths are 146.0 pm for a Si−H bond and 233 pm for a Si−Si bond. The structures of the silanes are analogues of the alkanes, starting with silane, SiH4, the analogue of methane, continuing with disilane Si2H6, the analogue of ethane, etc. They are mainly of theoretical or academic interest.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.

Plumbane is an inorganic chemical compound with the chemical formula PbH4. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom. Derivatives of plumbane include lead tetrafluoride, PbF4, and tetraethyllead, (CH3CH2)4Pb.

Chlorine azide is an inorganic compound that was discovered in 1908 by Friedrich Raschig. Concentrated ClN3 is notoriously unstable and may spontaneously detonate at any temperature.
Polysilicon hydrides are polymers containing only silicon and hydrogen. They have the formula where 0.2 ≤ n ≤ 2.5 and x is the number of monomer units. The polysilicon hydrides are generally colorless or pale-yellow/ocher powders that are easily hydrolyzed and ignite readily in air. The surfaces of silicon prepared by MOCVD using silane (SiH4) consist of a polysilicon hydride.
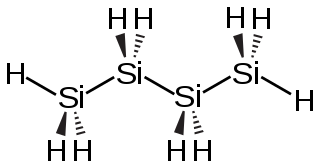
Tetrasilane is a silane with the structure formula SiH3–(SiH2)2–SiH3. It is the silane analog of butane.
Chlorotrifluorosilane is an inorganic gaseous compound with formula SiClF3 composed of silicon, fluorine and chlorine. It is a silane that substitutes hydrogen with fluorine and chlorine atoms.
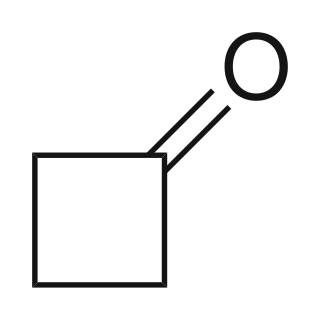
Cyclobutanone is an organic compound with molecular formula (CH2)3CO. It is a four-membered cyclic ketone (cycloalkanone). It is a colorless volatile liquid at room temperature. Since cyclopropanone is highly sensitive, cyclobutanone is the smallest easily handled cyclic ketone.

Sulfuryl diazide or sulfuryl azide is a chemical compound with the molecular formula SO2(N3)2. It was first described in the 1920s when its reactions with benzene and p-xylene were studied by Theodor Curtius and Karl Friedrich Schmidt. The compound is reported as having "exceedingly explosive, unpredictable properties" and "in many cases very violent explosions occurred without any apparent reason".

A silanide is a chemical compound containing an anionic silicon(IV) centre, the parent ion being SiH−3. The hydrogen atoms can also be substituted to produced more complex derivative anions such as tris(trimethylsilyl)silanide (hypersilyl), tris(tert-butyl)silanide, tris(pentafluoroethyl)silanide, or triphenylsilanide. The simple silanide ion can also be called trihydridosilanide or silyl hydride.
Protactinium(V) iodide is an inorganic compound, with the chemical formula of PaI5.