
Neptunium is a chemical element; it has symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. Like all actinides, it is radioactive, poisonous, pyrophoric, and capable of accumulating in bones, which makes the handling of neptunium dangerous.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.

A borane is a compound with the formula BxHy or a related anion. Many such boranes are known. Most common are those with 1 to 12 boron atoms. Although they have few practical applications, the boranes exhibit structures and bonding that differs strongly from the patterns seen in hydrocarbons. Hybrids of boranes and hydrocarbons, the carboranes are also well developed.
A substance is pyrophoric if it ignites spontaneously in air at or below 54 °C (129 °F) or within 5 minutes after coming into contact with air. Examples are organolithium compounds and triethylborane. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air. They can be handled safely in atmospheres of argon or nitrogen. Class D fire extinguishers are designated for use in fires involving pyrophoric materials. A related concept is hypergolicity, in which two compounds spontaneously ignite when mixed.
The Goldschmidt classification, developed by Victor Goldschmidt (1888–1947), is a geochemical classification which groups the chemical elements within the Earth according to their preferred host phases into lithophile (rock-loving), siderophile (iron-loving), chalcophile, and atmophile (gas-loving) or volatile.

Herbert Charles Brown was an American chemist and recipient of the 1979 Nobel Prize in Chemistry for his work with organoboranes.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen. Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation:
Plutonium hydride is a non-stoichiometric chemical compound with the formula PuH2+x. It is one of two characterized hydrides of plutonium; the other is PuH3. PuH2+x is non-stoichiometric with a composition range of PuH2 – PuH2.7. Metastable stoichiometries with an excess of hydrogen (PuH2.7 – PuH3) can also be formed. PuH2 has a cubic structure. It is readily formed from the elements at 1 atmosphere at 100–200°C: When the stoichiometry is close to PuH2 it has a silver appearance, but gets blacker as the hydrogen content increases, additionally the color change is associated with a reduction in conductivity.

Uranium(III) chloride, UCl3, is a water soluble salt of uranium. UCl3 is used mostly to reprocess spent nuclear fuel. Uranium(III) chloride is synthesized in various ways from uranium(IV) chloride; however, UCl3 is less stable than UCl4.
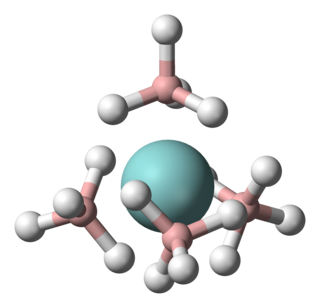
Uranium borohydride is the inorganic compound with the empirical formula U(BH4)4. Two polymeric forms are known, as well as a monomeric derivative that exists in the gas phase. Because the polymers convert to the gaseous form at mild temperatures, uranium borohydride once attracted much attention. It is solid green.
Uranium compounds are compounds formed by the element uranium (U). Although uranium is a radioactive actinide, its compounds are well studied due to its long half-life and its applications. It usually forms in the +4 and +6 oxidation states, although it can also form in other oxidation states.
Uranium hydride, also called uranium trihydride (UH3), is an inorganic compound and a hydride of uranium.
Binary compounds of hydrogen are binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types.

An iron hydride is a chemical system which contains iron and hydrogen in some associated form.
Mercury hydride may refer to:
Uranium(IV) hydride is a chemical compound with the chemical formula UH4, a metal hydride.
The Ames Project was a research and development project that was part of the larger Manhattan Project to build the first atomic bombs during World War II. It was founded by Frank Spedding from Iowa State College in Ames, Iowa as an offshoot of the Metallurgical Laboratory at the University of Chicago devoted to chemistry and metallurgy, but became a separate project in its own right. The Ames Project developed the Ames Process, a method for preparing pure uranium metal that the Manhattan Project needed for its atomic bombs and nuclear reactors. Between 1942 and 1945, it produced over 1,000 short tons (910 t) of uranium metal. It also developed methods of preparing and casting thorium, cerium and beryllium. In October 1945 Iowa State College received the Army-Navy "E" Award for Excellence in Production, an award usually only given to industrial organizations. In 1947 it became the Ames Laboratory, a national laboratory under the Atomic Energy Commission.
Group 14 hydrides are chemical compounds composed of hydrogen atoms and group 14 atoms.
Uranium iodide may refer to one of three chemical compounds:
Neodymium hydride may refer to:
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.







