In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity."
In chemistry, a reducing agent is a chemical species that "donates" an electron to an electron recipient.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.

Sodium amide, commonly called sodamide, is the inorganic compound with the formula NaNH2. It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is white, but commercial samples are typically gray due to the presence of small quantities of metallic iron from the manufacturing process. Such impurities do not usually affect the utility of the reagent. NaNH2 conducts electricity in the fused state, its conductance being similar to that of NaOH in a similar state. NaNH2 has been widely employed as a strong base in organic synthesis.
The chloralkali process is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide, which are commodity chemicals required by industry. Thirty five million tons of chlorine were prepared by this process in 1987. In 2022, this had increased to about 83 million tonnes. The chlorine and sodium hydroxide produced in this process are widely used in the chemical industry.

Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula NaBH4. It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodium borohydride is a reducing agent that finds application in papermaking and dye industries. It is also used as a reagent in organic synthesis.
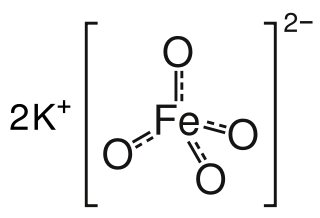
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.
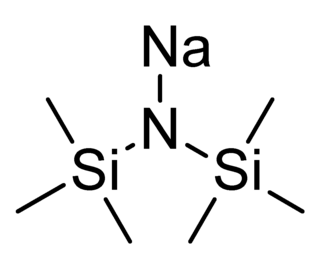
Sodium bis(trimethylsilyl)amide is the organosilicon compound with the formula NaN(Si 3)2. This species, usually called NaHMDS, is a strong base used for deprotonation reactions or base-catalyzed reactions. Its advantages are that it is commercially available as a solid and it is soluble not only in ethers, such as THF or diethyl ether, but also in aromatic solvents, like benzene and toluene by virtue of the lipophilic TMS groups.

The bisulfite ion (IUPAC-recommended nomenclature: hydrogensulfite) is the ion HSO−
3. Salts containing the HSO−
3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite (Na2S2O5). Sodium metabisulfite dissolves in water to give a solution of Na+HSO−
3.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.

Aluminium hydride is an inorganic compound with the formula AlH3. Alane and its derivatives are part of a family of common reducing reagents in organic synthesis based around group 13 hydrides. In solution—typically in ethereal solvents such tetrahydrofuran or diethyl ether—aluminium hydride forms complexes with Lewis bases, and reacts selectively with particular organic functional groups, and although it is not a reagent of choice, it can react with carbon-carbon multiple bonds. Given its density, and with hydrogen content on the order of 10% by weight, some forms of alane are, as of 2016, active candidates for storing hydrogen and so for power generation in fuel cell applications, including electric vehicles. As of 2006 it was noted that further research was required to identify an efficient, economical way to reverse the process, regenerating alane from spent aluminium product.
In electrochemistry, electrosynthesis is the synthesis of chemical compounds in an electrochemical cell. Compared to ordinary redox reactions, electrosynthesis sometimes offers improved selectivity and yields. Electrosynthesis is actively studied as a science and also has industrial applications. Electrooxidation has potential for wastewater treatment as well.

Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g., with tetrahydrofuran or dimethoxyethane, which bind to the sodium cation. An oxygen-sensitive colourless solid, it is a reagent in organometallic and organic chemical research. The dioxane solvated sodium salt is known as Collman's reagent, in recognition of James P. Collman, an early popularizer of its use.
The Castner–Kellner process is a method of electrolysis on an aqueous alkali chloride solution to produce the corresponding alkali hydroxide, invented by American Hamilton Castner and Austrian Carl Kellner in the 1890s. It is a type of chloralkali process, but in this role it is gradually being replaced by membrane electrolysis which has lower energy cost and fewer environmental concerns.

Sodium methylsulfinylmethylide is the sodium salt of the conjugate base of dimethyl sulfoxide. This unusual salt has some uses in organic chemistry as a base and nucleophile.

Chloroauric acid is an inorganic compound with the chemical formula H[AuCl4]. It forms hydrates H[AuCl4]·nH2O. Both the trihydrate and tetrahydrate are known. Both are orange-yellow solids consisting of the planar [AuCl4]− anion. Often chloroauric acid is handled as a solution, such as those obtained by dissolution of gold in aqua regia. These solutions can be converted to other gold complexes or reduced to metallic gold or gold nanoparticles.
Chlorine gas can be produced by extracting from natural materials, including the electrolysis of a sodium chloride solution (brine) and other ways.

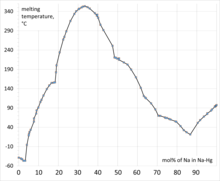
An amalgam is an alloy of mercury with another metal. It may be a liquid, a soft paste or a solid, depending upon the proportion of mercury. These alloys are formed through metallic bonding, with the electrostatic attractive force of the conduction electrons working to bind all the positively charged metal ions together into a crystal lattice structure. Almost all metals can form amalgams with mercury, the notable exceptions being iron, platinum, tungsten, and tantalum. Silver-mercury amalgams are important in dentistry, and gold-mercury amalgam is used in the extraction of gold from ore. Dentistry has used alloys of mercury with metals such as silver, copper, indium, tin and zinc.
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity.