
Diphosgene is an organic chemical compound with the formula ClCO2CCl3. This colorless liquid is a valuable reagent in the synthesis of organic compounds. Diphosgene is related to phosgene and has comparable toxicity, but is more conveniently handled because it is a liquid, whereas phosgene is a gas.
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is R−:C−R' or R=C: where the R represents substituents or hydrogen atoms.

Tetrabutylammonium hydroxide is the chemical compound with the formula (C4H9)4NOH, abbreviated Bu4NOH with the acronym TBAOH or TBAH. This species is employed as a solution in water or alcohols. It is a common base in organic chemistry. Relative to more conventional inorganic bases, such as KOH and NaOH, Bu4NOH is more soluble in organic solvents.
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds.
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive towards water.

Vanadium oxytrichloride is the inorganic compound with the formula VOCl3. This yellow distillable liquid hydrolyzes readily in air. It is an oxidizing agent. It is used as a reagent in organic synthesis. Samples often appear red or orange owing to an impurity of vanadium tetrachloride.

The Petasis reagent, named after Nicos A. Petasis, is an organotitanium compound with the formula Cp2Ti(CH3)2. It is an orange-colored solid.

Tungsten hexachloride is an inorganic chemical compound of tungsten and chlorine with the chemical formula WCl6. This dark violet-blue compound exists as volatile crystals under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. Other examples of charge-neutral hexachlorides are rhenium(VI) chloride and molybdenum(VI) chloride. The highly volatile tungsten hexafluoride is also known.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Many organomercury compounds are highly toxic, but some are used in medicine, e.g., merbromin ("Mercurochrome") and the vaccine preservative thiomersal.

Allylpalladium(II) chloride dimer (APC) is a chemical compound with the formula [(η3-C3H5)PdCl]2. This yellow air-stable compound is an important catalyst used in organic synthesis. It is one of the most widely used transition metal allyl complexes.

Diethylaminosulfur trifluoride (DAST) is the organosulfur compound with the formula Et2NSF3. This liquid is a fluorinating reagent used for the synthesis of organofluorine compounds. The compound is colourless; older samples assume an orange colour.

Triethyl phosphite (TEP) is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis.

K-selectride is the organoboron compound with the formula KBH(C4H9)3. It is the potassium salt of tri(sec-butyl)borohydride. The compound is sold as solution in THF. It is a strong reducing agent. Solutions are pyrophoric and highly reactive toward water and protic compounds. It is handled using air-free technique. It is produced by the reaction of tri(sec-butyl)borane with potassium hydride. The reagent is used to reduce ketones to alcohols.

Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.
Zinc amalgam is a solution of zinc in mercury. In practice the term refers to particles of zinc with a surface coating of the amalgam. A gray solid, it is typically used for reduction. It is written as Zn(Hg) in reactions. It is usually prepared by treating an aqueous suspension of zinc with mercuric chloride. Some zinc chloride is produced in the process.
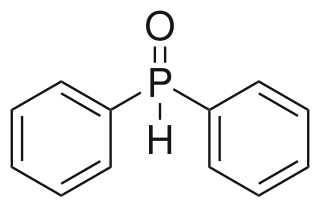
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents.

Tetrachlorocyclopropene is a chemical compound with the formula C3Cl4. A colorless liquid, the compound is a reagent used to prepare acetylene derivatives and in organic synthesis. It is prepared by addition of dichlorocarbene to trichloroethylene. It can react with water and alcohols rapidly.
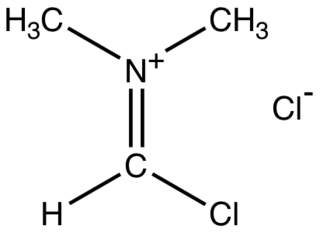
The Vilsmeier reagent is an organic compound with the formula [(CH3)2NCHCl]Cl. It is a salt consisting of the N,N-dimethyliminium cation ([(CH3)2N=CHCl]+) and chloride anion. Depending on the particular reaction, the anion can vary. In typical POCl3-based reactions, the anion is PO2Cl2−. The iminium cation [(CH3)2N=CHCl]+ is the reactive component of interest. This iminium species is a derivative of the imidoyl chloride CH3N=CHCl. Analogues of this particular reagent are generated when tertiary amides other than DMF are treated with POCl3.
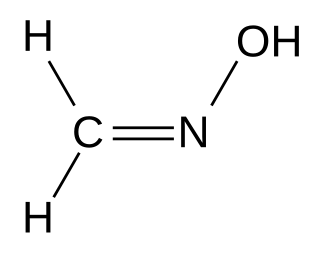
Formaldoxime is the organic compound with the formula H2C=N−OH. It is the oxime of formaldehyde. A colorless liquid, the pure compound tends to polymerize into a cyclic trimer. Aqueous solutions are stable as is the formaldoxime hydrochloride. It is a reagent in organic synthesis for the conversion of aryl diazonium salts to aryl aldehydes.
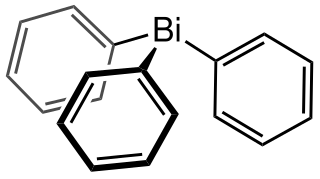
Triphenylbismuthine is an organobismuth compound with the formula Bi(C6H5)3. It is a white, air-stable solid that is soluble in organic solvents. It is prepared by treatment of bismuth trichloride with phenylmagnesium bromide.



















