
Zinc carbonate is the inorganic compound with the formula ZnCO3. It is a white solid that is insoluble in water. It exists in nature as the mineral smithsonite. It is prepared by treating cold solutions of zinc sulfate with potassium bicarbonate. Upon warming, it converts to basic zinc carbonate (Zn5(CO3)2(OH)6).
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Mercury(I) chloride is the chemical compound with the formula Hg2Cl2. Also known as the mineral calomel (a rare mineral) or mercurous chloride, this dense white or yellowish-white, odorless solid is the principal example of a mercury(I) compound. It is a component of reference electrodes in electrochemistry.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and the hemipentahydrate CdCl2•2.5H2O.

Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.

Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water from the atmosphere. It has no major application.
The bead test is a traditional part of qualitative inorganic analysis to test for the presence of certain metals. The oldest one is the borax bead test or blister test. It was introduced by Berzelius in 1812. Since then other salts were used as fluxing agents, such as sodium carbonate or sodium fluoride. The most important one after borax is microcosmic salt, which is the basis of the microcosmic salt bead test.

Mercury(II) cyanide, also known as mercuric cyanide, is a poisonous compound of mercury and cyanide. It is an odorless, toxic white powder. It is highly soluble in polar solvents such as water, alcohol, and ammonia, slightly soluble in ether, and insoluble in benzene and other hydrophobic solvents.

Sodium hexanitritocobaltate(III) is inorganic compound with the formula Na3[Co(NO2)6]. The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO2)6]3−. It was a reagent for the qualitative test for potassium and ammonium ions.

Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).
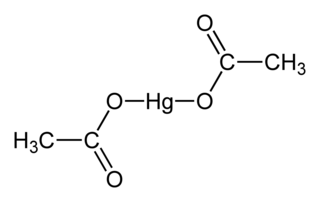
Mercury(II) acetate, also known as mercuric acetate is a chemical compound, the mercury(II) salt of acetic acid, with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.
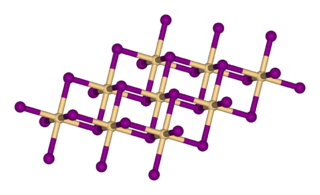
Cadmium iodide is an inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects.
Germanium iodides are inorganic compound with the formula GeIx. Two such compounds exist: germanium(II) iodide, GeI2, and germanium(IV) iodide GeI4.

Mercury(II) bromide or mercuric bromide is an inorganic compound with the formula HgBr2. This white solid is a laboratory reagent. Like all mercury salts, it is highly toxic.
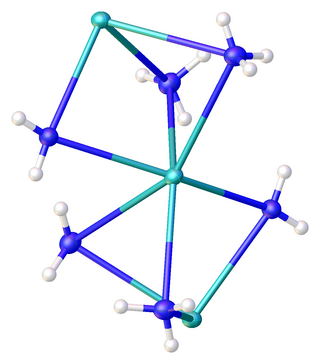
Potassium amide is an inorganic compound with the chemical formula KNH2. Like other alkali metal amides, it is a white solid that hydrolyzes readily. It is a strong base.
In chemistry and physics, the iron group refers to elements that are in some way related to iron; mostly in period (row) 4 of the periodic table. The term has different meanings in different contexts.

In organic chemistry, borate esters are organoboron compounds which are conveniently prepared by the stoichiometric condensation reaction of boric acid with alcohols. There are two main classes of borate esters: orthoborates, B(OR)3 and metaborates, B3O3(OR)3. Metaborates contain 6-membered boroxine rings.

Arthur Israel Vogel was a British chemist known for his Chemistry textbooks. He became the head of the chemistry department at Woolwich Polytechnic at the age of 27.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.
















