 | |
| Names | |
|---|---|
| IUPAC name Mercuric azanide chloride | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.030.292 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
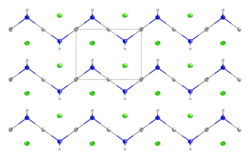
| Hg(NH2)Cl | |
| Molar mass | 252.07 g·mol−1 |
| Appearance | White powder of small prisms [1] |
| Odor | None [2] |
| Density | 5.7 g/cm3 (at 20 °C (68 °F; 293 K)) [1] |
| Boiling point | Sublimes [3] |
| 1.4 g/L (cold[ quantify ]); decomposes if hot [1] | |
| Solubility | Soluble in sodium thiosulfate or ammonium carbonate solution. [3] [2] |
| Solubility in nitric acid | soluble in warm |
| Solubility in hydrochloric acid | soluble in warm |
| Solubility in acetic acid | soluble in warm |
| Pharmacology | |
| D08AK01 ( WHO ) | |
| Hazards | |
| GHS labelling: [4] | |
   | |
| Danger | |
| H300+H310+H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310+P330, P302+P350+P310, P304+P340+P310, P314, P362, P391, P403+P233, P405, P501 | |
| Flash point | Non-combustible |
Threshold limit value (TLV) | 0.025 mg/m3 [5] (TWA) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
LC50 (median concentration) | 0.051 mg/L (inhalation, 4h, dust/mist) [4] |
| NIOSH (US health exposure limits): [6] | |
PEL (Permissible) | 0.1 mg/m3 (TWA) |
REL (Recommended) | 0.1 mg/m3 (C, skin, as Hg) |
IDLH (Immediate danger) | 10 mg/m3 (as Hg) |
| Related compounds | |
Related compounds | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Mercuric amidochloride is an inorganic compound with the formula Hg(N H 2)Cl .
