
Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble barium salts, it is a white powder, highly toxic, and imparts a yellow-green coloration to a flame. It is also hygroscopic, converting to the dihydrate BaCl2·2H2O, which are colourless crystals with a bitter salty taste. It has limited use in the laboratory and industry.

Phosphorus pentabromide is a reactive, yellow solid of formula PBr5, which has the structure [PBr4]+Br− in the solid state but in the vapor phase is completely dissociated to PBr3 and Br2. Rapid cooling of this phase to 15 K leads to formation of the ionic species phosphorus heptabromide.
Tin(II) bromide is a chemical compound of tin and bromine with a chemical formula of SnBr2. Tin is in the +2 oxidation state. The stability of tin compounds in this oxidation state is attributed to the inert pair effect.
The thallium halides include monohalides, where thallium has oxidation state +1, trihalides in which thallium generally has oxidation state +3, and some intermediate halides containing thallium with mixed +1 and +3 oxidation states. These materials find use in specialized optical settings, such as focusing elements in research spectrophotometers. Compared to the more common zinc selenide-based optics, materials such as thallium bromoiodide enable transmission at longer wavelengths. In the infrared, this allows for measurements as low as 350 cm−1 (28 μm), whereas zinc selenide is opaque by 21.5 μm, and ZnSe optics are generally only usable to 650 cm−1 (15 μm).
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.
There are three sets of gallium halides, the trihalides where gallium has oxidation state +3, the intermediate halides containing gallium in oxidation states +1, +2 and +3 and some unstable monohalides, where gallium has oxidation state +1.

Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its properties are similar to those of aluminium chloride, due to beryllium's diagonal relationship with aluminium.

Indium(I) bromide is a chemical compound of indium and bromine. It is a red crystalline compound that is isostructural with β-TlI and has a distorted rock salt structure. Indium(I) bromide is generally made from the elements, heating indium metal with InBr3. It has been used in the sulfur lamp. In organic chemistry, it has been found to promote the coupling of α, α-dichloroketones to 1-aryl-butane-1,4-diones. Oxidative addition reactions with for example alkyl halides to give alkyl indium halides and with NiBr complexes to give Ni-In bonds are known. It is unstable in water decomposing into indium metal and indium tribromide. When indium dibromide is dissolved in water, InBr is produced as a, presumably, insoluble red precipitate, that then rapidly decomposes.

Cobalt(III) oxide is the inorganic compound with the formula of Co2O3. Although only two oxides of cobalt are well characterized, CoO and Co3O4, procedures claiming to give Co2O3 have been described. Thus treatment of Co(II) salts such as cobalt(II) sulfate with an aqueous solution of sodium hypochlorite (also known as bleach) gives a black solid:

Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature.

Bismuth tribromide is an inorganic compound of bismuth and bromine with the chemical formula BiBr3.
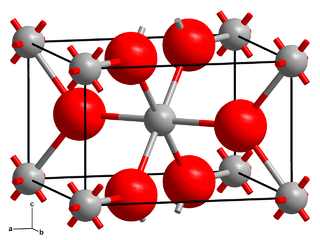
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.
Rhenium compounds are compounds formed by the transition metal rhenium (Re). Rhenium can form in many oxidation states, and compounds are known for every oxidation state from -3 to +7 except -2, although the oxidation states +7, +4, and +3 are the most common. Rhenium is most available commercially as salts of perrhenate, including sodium and ammonium perrhenates. These are white, water-soluble compounds. The tetrathioperrhenate anion [ReS4]− is possible.

Thiophosphoryl bromide is an inorganic compound with the formula PSBr3.


















