
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 124 years half-life of polonium-209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.
Livermorium is a synthetic chemical element; it has symbol Lv and atomic number 116. It is an extremely radioactive element that has only been created in a laboratory setting and has not been observed in nature. The element is named after the Lawrence Livermore National Laboratory in the United States, which collaborated with the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, to discover livermorium during experiments conducted between 2000 and 2006. The name of the laboratory refers to the city of Livermore, California, where it is located, which in turn was named after the rancher and landowner Robert Livermore. The name was adopted by IUPAC on May 30, 2012. Five isotopes of livermorium are known, with mass numbers of 288 and 290–293 inclusive; the longest-lived among them is livermorium-293 with a half-life of about 60 milliseconds. A sixth possible isotope with mass number 294 has been reported but not yet confirmed.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are also called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
The slow neutron-capture process, or s-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The s-process is responsible for the creation (nucleosynthesis) of approximately half the atomic nuclei heavier than iron.
The inert-pair effect is the tendency of the two electrons in the outermost atomic s-orbital to remain unshared in compounds of post-transition metals. The term inert-pair effect is often used in relation to the increasing stability of oxidation states that are two less than the group valency for the heavier elements of groups 13, 14, 15 and 16. The term "inert pair" was first proposed by Nevil Sidgwick in 1927. The name suggests that the outermost s electron pairs are more tightly bound to the nucleus in these atoms, and therefore more difficult to ionize or share.
Polonium-210 (210Po, Po-210, historically radium F) is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days (about 4+1⁄2 months), the longest half-life of all naturally occurring polonium isotopes (210–218Po). First identified in 1898, and also marking the discovery of the element polonium, 210Po is generated in the decay chain of uranium-238 and radium-226. 210Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, mostly due to alpha particles, which easily cause radiation damage, including cancer in surrounding tissue. The specific activity of 210
Po is 166 TBq/g, i.e., 1.66 × 1014 Bq/g. At the same time, 210Po is not readily detected by common radiation detectors, because its gamma rays have a very low energy. Therefore, 210
Po can be considered as a quasi-pure alpha emitter.
In chemistry, a plumbate often refers to compounds that can be viewed as derivatives of the hypothetical PbO2−3 anion.
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals.
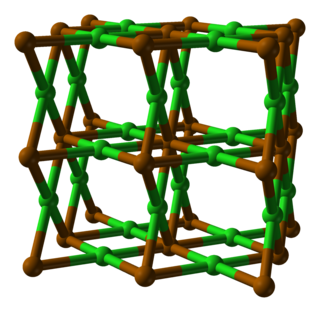
Polonium dichloride is a chemical compound of the radioactive metalloid, polonium and chlorine. Its chemical formula is PoCl2. It is an ionic salt.
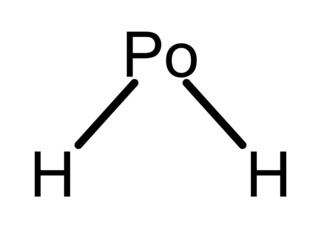
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen. It is a volatile and very labile compound, from which many polonides can be derived. Additionally, it is radioactive.

A polonide is a chemical compound of the radioactive element polonium with any element less electronegative than polonium. Polonides are usually prepared by a direct reaction between the elements at temperatures of around 300–400 °C. They are amongst the most chemically stable compounds of polonium, and can be divided into two broad groups:

Magnesium polonide (MgPo) is a salt of magnesium and polonium. It is a polonide, a set of very chemically stable compounds of polonium.

Sodium polonide is a radioactive chemical compound with the formula Na2Po. This salt is a polonide, a set of very chemically stable compounds of polonium. Due to the difference in electronegativity (ΔEN) between sodium and polonium and the slight non-metallic character of polonium, it is intermediate between intermetallic phases and ionic compounds.

Potassium polonide is a chemical compound with the formula K2Po. It is a polonide, a set of very chemically stable compounds of polonium.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.

Monofluorophosphate is an anion with the formula PO3F2−, which is a phosphate group with one oxygen atom substituted with a fluoride atom. The charge of the ion is −2. The ion resembles sulfate in size, shape and charge, and can thus form compounds with the same structure as sulfates. These include Tutton's salts and langbeinites. The most well-known compound of monofluorophosphate is sodium monofluorophosphate, commonly used in toothpaste.
Oxyphosphides are chemical compounds formally containing the group PO, with one phosphorus and one oxygen atom. The phosphorus and oxygen are not bound together as in phosphates or phosphine oxides, instead they are bound separately to the cations (metals), and could be considered as a mixed phosphide-oxide compound. So a compound with OmPn requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxy-pnictide compounds.

Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species.
Organopolonium chemistry describes the synthesis and properties of chemical compounds containing a carbon to polonium chemical bond.









