
Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 124 years half-life of polonium-209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

In crystallography, the cubiccrystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
Magnesium peroxide (MgO2) is an odorless fine powder peroxide with a white to off-white color. It is similar to calcium peroxide because magnesium peroxide also releases oxygen by breaking down at a controlled rate with water. Commercially, magnesium peroxide often exists as a compound of magnesium peroxide and magnesium hydroxide.
Post-perovskite (pPv) is a high-pressure phase of magnesium silicate (MgSiO3). It is composed of the prime oxide constituents of the Earth's rocky mantle (MgO and SiO2), and its pressure and temperature for stability imply that it is likely to occur in portions of the lowermost few hundred km of Earth's mantle.

Orthocarbonic acid, carbon hydroxide, methanetetrol is the name given to a hypothetical compound with the chemical formula H4CO4 or C(OH)4. Its molecular structure consists of a single carbon atom bonded to four hydroxyl groups. It would be therefore a fourfold alcohol. In theory it could lose four protons to give the hypothetical oxocarbon anion orthocarbonateCO4−4, and is therefore considered an oxoacid of carbon.

Heusler compounds are magnetic intermetallics with face-centered cubic crystal structure and a composition of XYZ (half-Heuslers) or X2YZ (full-Heuslers), where X and Y are transition metals and Z is in the p-block. The term derives from the name of German mining engineer and chemist Friedrich Heusler, who studied such a compound (Cu2MnAl) in 1903. Many of these compounds exhibit properties relevant to spintronics, such as magnetoresistance, variations of the Hall effect, ferro-, antiferro-, and ferrimagnetism, half- and semimetallicity, semiconductivity with spin filter ability, superconductivity, topological band structure and are actively studied as thermoelectric materials. Their magnetism results from a double-exchange mechanism between neighboring magnetic ions. Manganese, which sits at the body centers of the cubic structure, was the magnetic ion in the first Heusler compound discovered. (See the Bethe–Slater curve for details of why this happens.)
In crystallography, polymorphism is the phenomenon where a compound or element can crystallize into more than one crystal structure.

The A15 phases (also known as β-W or Cr3Si structure types) are series of intermetallic compounds with the chemical formula A3B (where A is a transition metal and B can be any element) and a specific structure. The A15 phase is also one of the members in the Frank–Kasper phases family. Many of these compounds have superconductivity at around 20 K (−253 °C; −424 °F), which is comparatively high, and remain superconductive in magnetic fields of tens of teslas (hundreds of kilogauss). This kind of superconductivity (Type-II superconductivity) is an important area of study as it has several practical applications.

Calcium disilicide (CaSi2) is an inorganic compound, a silicide of calcium. It is a whitish or dark grey to black solid matter with melting point 1033 °C. It is insoluble in water, but may decompose when subjected to moisture, evolving hydrogen and producing calcium hydroxide. It decomposes in hot water, and is flammable and may ignite spontaneously in air.

Thaumasite is a calcium silicate mineral, containing Si atoms in unusual octahedral configuration, with chemical formula Ca3Si(OH)6(CO3)(SO4)·12H2O, also sometimes more simply written as CaSiO3·CaCO3·CaSO4·15H2O.
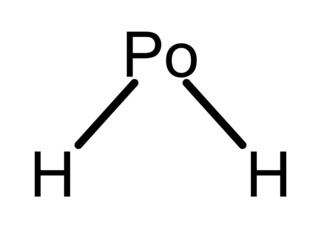
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen. It is a volatile and very labile compound, from which many polonides can be derived. Additionally, it is radioactive.

A polonide is a chemical compound of the radioactive element polonium with any element less electronegative than polonium. Polonides are usually prepared by a direct reaction between the elements at temperatures of around 300–400 °C. They are amongst the most chemically stable compounds of polonium, and can be divided into two broad groups:

Magnesium polonide (MgPo) is a salt of magnesium and polonium. It is a polonide, a set of very chemically stable compounds of polonium.

Sodium polonide is a radioactive chemical compound with the formula Na2Po. This salt is a polonide, a set of very chemically stable compounds of polonium. Due to the difference in electronegativity (ΔEN) between sodium and polonium and the slight non-metallic character of polonium, it is intermediate between intermetallic phases and ionic compounds.

Potassium polonide is a chemical compound with the formula K2Po. It is a polonide, a set of very chemically stable compounds of polonium.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.

Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material.
Oxybismuthides or bismuthide oxides are chemical compounds formally containing the group BiO, with one bismuth and one oxygen atom. The bismuth and oxygen are not bound together as in bismuthates, instead they make a separate presence bound to the cations (metals), and could be considered as a mixed bismuthide-oxide compound. So a compound with OmBin requires cations to balance a negative charge of 2m+3n. The cations will have charges of +2 or +3. The trications are often rare earth elements or actinides. They are in the category of oxypnictide compounds.

Cobalt germanide (CoGe) is an intermetallic compound, a germanide of cobalt.

Lanthanum phosphide is an inorganic compound of lanthanum and phosphorus with the chemical formula LaP.














