
Polonium is a chemical element with the symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 125.2 years half-life of polonium 209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

In chemistry, a hydride is formally the anion of hydrogen (H−), a hydrogen atom with two electrons. The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed.
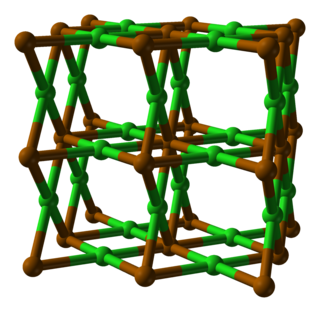
Polonium dichloride is a chemical compound of the radioactive metalloid, polonium and chlorine. Its chemical formula is PoCl2. It is an ionic salt.
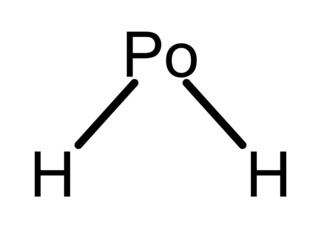
Polonium hydride (also known as polonium dihydride, hydrogen polonide, or polane) is a chemical compound with the formula PoH2. It is a liquid at room temperature, the second hydrogen chalcogenide with this property after water. It is very unstable chemically and tends to decompose into elemental polonium and hydrogen. It is a volatile and very labile compound, from which many polonides can be derived. Additionally, like all polonium compounds, it is highly radioactive.

A polonide is a chemical compound of the radioactive element polonium with any element less electronegative than polonium. Polonides are usually prepared by a direct reaction between the elements at temperatures of around 300–400 °C. They are amongst the most chemically stable compounds of polonium, and can be divided into two broad groups:

Polonium hexafluoride is a possible chemical compound of polonium and fluorine and one of the seventeen known binary hexafluorides.
Polonium tetrachloride (also known as polonium(IV) chloride) is a chemical compound with the formula PoCl4. The salt is a hygroscopic bright yellow crystalline solid at room temperature. Above 200 °C, it tends to decompose into polonium dichloride and excess chlorine, similar to selenium tetrachloride and tellurium tetrachloride.

Polonium dioxide (also known as polonium(IV) oxide) is a chemical compound with the formula PoO2. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium trioxide (PoO3). It is a pale yellow crystalline solid at room temperature. Under lowered pressure (such as a vacuum), it decomposes into elemental polonium and oxygen at 500 °C. It is the most stable oxide of polonium and is an interchalcogen.
Polonium trioxide (also known as polonium(VI) oxide) is a chemical compound with the formula PoO3. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium dioxide (PoO2). It is an interchalcogen that has so far only been detected in trace amounts.
Polonium monoxide is a chemical compound with the formula PoO. It is one of three oxides of polonium, the other two being polonium dioxide and polonium trioxide. It is an interchalcogen.
Polonium dibromide (also known as polonium(II) bromide) is a chemical compound with the formula PoBr2. This salt is a purple-brown crystalline solid at room temperature. It sublimes (decomposing slightly) at 110 °C/30 μ and decomposes when melted in nitrogen gas at 270–280 °C.
Lithium polonide is a chemical compound with the formula Li2Po. It is a polonide, a set of very chemically stable compounds of polonium.
Sodium polonide is a radioactive chemical compound with the formula Na2Po. This salt is a polonide, a set of very chemically stable compounds of polonium. Due to the difference in electronegativity (ΔEN) between sodium and polonium and the slight non-metallic character of polonium, it is intermediate between intermetallic phases and ionic compounds.
Potassium polonide is a chemical compound with the formula K2Po. It is a polonide, a set of very chemically stable compounds of polonium.

The metallic elements in the periodic table located between the transition metals to their left and the chemically weak nonmetallic metalloids to their right have received many names in the literature, such as post-transition metals, poor metals, other metals, p-block metals and chemically weak metals. The most common name, post-transition metals, is generally used in this article.
Organopolonium chemistry describes the synthesis and properties of chemical compounds containing a carbon to polonium chemical bond.
Calcium polonide is an intermetallic compound with the chemical formula CaPo. It is made up of calcium and polonium. Rather than being found in nature, the compound is entirely synthetic, and difficult to study, due to polonium's high vapor pressure, radioactivity, and easy oxidation in air.
Polonium tetranitrate is an inorganic compound, a salt of polonium and nitric acid with the chemical formula Po(NO3)4. The compound is radioactive, forms white crystals.
Polonium sulfide is an inorganic compound of polonium and sulfur with the chemical formula PoS. The compound is radioactive and forms black crystals.
Polonium tetraiodide is a binary inorganic compound of polonium and iodine with the chemical formula PoI
4. The compound forms volatile black crystals.








