Aqua regia is a mixture of nitric acid and hydrochloric acid, optimally in a molar ratio of 1:3. Aqua regia is a fuming liquid. Freshly prepared aqua regia is colorless, but it turns yellow, orange or red within seconds from the formation of nitrosyl chloride and nitrogen dioxide. It was so named by alchemists because it can dissolve noble metals like gold and platinum, though not all metals.
The term chloride refers to a compound or molecule that contains either a chlorine ion, which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond. Many inorganic chlorides are salts. Many organic compounds are chlorides. The pronunciation of the word "chloride" is.

Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.

Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It is a product of copper mining and the precursor to many other copper-containing products and chemical compounds.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.

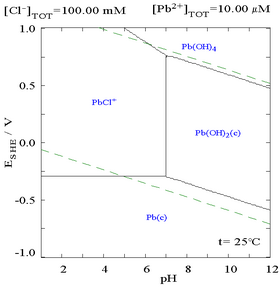
Lead(IV) oxide, commonly known as lead dioxide, is an inorganic compound with the chemical formula PbO2. It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. It has several important applications in electrochemistry, in particular as the positive plate of lead acid batteries.
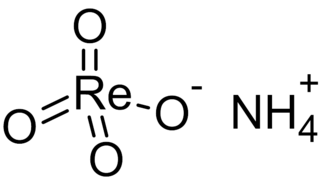
Ammonium perrhenate (APR) is the ammonium salt of perrhenic acid, NH4ReO4. It is the most common form in which rhenium is traded. It is a white salt; soluble in ethanol and water, and mildly soluble in NH4Cl. It was first described soon after the discovery of rhenium.

Kipp's apparatus, also called a Kipp generator, is an apparatus designed for preparation of small volumes of gases. It was invented around 1844 by the Dutch pharmacist Petrus Jacobus Kipp and widely used in chemical laboratories and for demonstrations in schools into the second half of the 20th century.
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.

Gold compounds are compounds by the element gold (Au). Although gold is the most noble of the noble metals, it still forms many diverse compounds. The oxidation state of gold in its compounds ranges from −1 to +5, but Au(I) and Au(III) dominate its chemistry. Au(I), referred to as the aurous ion, is the most common oxidation state with soft ligands such as thioethers, thiolates, and organophosphines. Au(I) compounds are typically linear. A good example is Au(CN)−2, which is the soluble form of gold encountered in mining. The binary gold halides, such as AuCl, form zigzag polymeric chains, again featuring linear coordination at Au. Most drugs based on gold are Au(I) derivatives.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.

Silver is a relatively unreactive metal, although it can form several compounds. The common oxidation states of silver are (in order of commonness): +1 (the most stable state; for example, silver nitrate, AgNO3); +2 (highly oxidising; for example, silver(II) fluoride, AgF2); and even very rarely +3 (extreme oxidising; for example, potassium tetrafluoroargentate(III), KAgF4). The +3 state requires very strong oxidising agents to attain, such as fluorine or peroxodisulfate, and some silver(III) compounds react with atmospheric moisture and attack glass. Indeed, silver(III) fluoride is usually obtained by reacting silver or silver monofluoride with the strongest known oxidizing agent, krypton difluoride.

Arsenic pentasulfide is an inorganic compound containing arsenic and sulfur.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.

Aluminium (British and IUPAC spellings) or aluminum (North American spelling) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances. Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency; this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship. However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus filled d- and f-subshells. Hence, aluminium does not suffer the effects of incomplete shielding of valence electrons by inner electrons from the nucleus that its heavier congeners do. Aluminium's electropositive behavior, high affinity for oxygen, and highly negative standard electrode potential are all more similar to those of scandium, yttrium, lanthanum, and actinium, which have ds2 configurations of three valence electrons outside a noble gas core: aluminium is the most electropositive metal in its group. Aluminium also bears minor similarities to the metalloid boron in the same group; AlX3 compounds are valence isoelectronic to BX3 compounds (they have the same valence electronic structure), and both behave as Lewis acids and readily form adducts. Additionally, one of the main motifs of boron chemistry is regular icosahedral structures, and aluminium forms an important part of many icosahedral quasicrystal alloys, including the Al–Zn–Mg class.
Gallium compounds are compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2. There are also compounds of gallium with negative oxidation states, ranging from -5 to -1, most of these compounds being magnesium gallides (MgxGay).
Neptunium compounds are compounds containing the element neptunium (Np). Neptunium has five ionic oxidation states ranging from +3 to +7 when forming chemical compounds, which can be simultaneously observed in solutions. It is the heaviest actinide that can lose all its valence electrons in a stable compound. The most stable state in solution is +5, but the valence +4 is preferred in solid neptunium compounds. Neptunium metal is very reactive. Ions of neptunium are prone to hydrolysis and formation of coordination compounds.

Bismuthyl — inorganic oxygen-containing singly charged ion with the chemical formula BiO+, is an oxycation of bismuth in the +3 oxidation state. Most often it is formed during the hydrolysis of trivalent bismuth salts, primarily nitrate, chloride and other halides. In chemical compounds, bismuthyl plays the role of a monovalent cation.