
Chromium is a chemical element; it has symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
Chromic acid is an inorganic acid composed of the elements chromium, oxygen, and hydrogen. It is a dark, purplish red, odorless, sand-like solid powder. When dissolved in water, it is a strong acid. There are 2 types of chromic acid: molecular chromic acid with the formula H
2CrO
4 and dichromic acid with the formula H
2Cr
2O
7.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Pyridinium chlorochromate (PCC) is a yellow-orange salt with the formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for oxidation of alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective.

Chromium trioxide (also known as chromium(VI) oxide or chromic anhydride) is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Potassium chromate is the inorganic compound with the formula K2CrO4. This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds.

Caesium chromate or cesium chromate is an inorganic compound with the formula Cs2CrO4. It is a yellow crystalline solid that is the caesium salt of chromic acid, and it crystallises in the orthorhombic system.
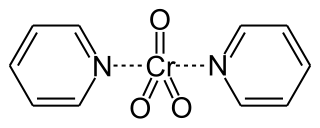
Collins reagent is the complex of chromium(VI) oxide with pyridine in dichloromethane. This metal-pyridine complex, a red solid, is used to oxidize primary alcohols to the corresponding aldehydes and secondary alcohols to the corresponding ketones. This complex is a hygroscopic orange solid.

Potassium chlorochromate is an inorganic compound with the formula KCrO3Cl. It is the potassium salt of chlorochromate, [CrO3Cl]−. It is a water-soluble orange compound is used occasionally for oxidation of organic compounds. It is sometimes called Péligot's salt, in recognition of its discoverer Eugène-Melchior Péligot.

Chromium(III) sulfate usually refers to the inorganic compounds with the formula Cr2(SO4)3.x(H2O), where x can range from 0 to 18. Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores.

Chromium(VI) peroxide or chromium oxide peroxide is an unstable compound with the formula CrO5. This compound contains one oxo ligand and two peroxo ligands, making a total of five oxygen atoms per chromium atom.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.

Chromyl fluoride is an inorganic compound with the formula CrO2F2. It is a violet-red colored crystalline solid that melts to an orange-red liquid.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.























