The actinide or actinoid series encompasses the 14 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Berkelium is a synthetic chemical element; it has symbol Bk and atomic number 97. It is a member of the actinide and transuranium element series. It is named after the city of Berkeley, California, the location of the Lawrence Berkeley National Laboratory where it was discovered in December 1949. Berkelium was the fifth transuranium element discovered after neptunium, plutonium, curium and americium.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Californium is a synthetic chemical element; it has symbol Cf and atomic number 98. The element was first synthesized in 1950 at Lawrence Berkeley National Laboratory, by bombarding curium with alpha particles. It is an actinide element, the sixth transuranium element to be synthesized, and has the second-highest atomic mass of all elements that have been produced in amounts large enough to see with the naked eye. The element was named after the university and the U.S. state of California.

Einsteinium is a synthetic chemical element; it has symbol Es and atomic number 99. Einsteinium is a member of the actinide series and it is the seventh transuranium element. It was named in honor of Albert Einstein.

Few compounds of californium have been made and studied. The only californium ion that is stable in aqueous solutions is the californium(III) cation. The other two oxidation states are IV (strong oxidizing agents) and II (strong reducing agents). The element forms a water-soluble chloride, nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate or hydroxide. If problems of availability of the element could be overcome, then CfBr2 and CfI2 would likely be stable.

Berkelium forms a number of chemical compounds, where it normally exists in an oxidation state of +3 or +4, and behaves similarly to its lanthanide analogue, terbium. Like all actinides, berkelium easily dissolves in various aqueous inorganic acids, liberating gaseous hydrogen and converting into the trivalent oxidation state. This trivalent state is the most stable, especially in aqueous solutions, but tetravalent berkelium compounds are also known. The existence of divalent berkelium salts is uncertain and has only been reported in mixed lanthanum chloride-strontium chloride melts. Aqueous solutions of Bk3+ ions are green in most acids. The color of the Bk4+ ions is yellow in hydrochloric acid and orange-yellow in sulfuric acid. Berkelium does not react rapidly with oxygen at room temperature, possibly due to the formation of a protective oxide surface layer; however, it reacts with molten metals, hydrogen, halogens, chalcogens and pnictogens to form various binary compounds. Berkelium can also form several organometallic compounds.
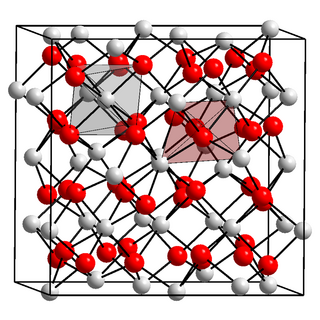
Einsteinium(III) oxide is an oxide of the synthetic actinide einsteinium which has the molecular formula Es2O3. It is a colourless solid.

Curium(III) fluoride or curium trifluoride is the chemical compound composed of curium and fluorine with the formula CmF3. It is a white, nearly insoluble salt that has the same crystal structure as LaF3. It precipitates as a hydrate when fluoride ions are added to a weakly acidic Cm(III) solution; alternatively it can be synthesized by reacting hydrofluoric acid with Cm(OH)3. The anhydrous form is then obtained by desiccation or by treatment with hydrogen fluoride gas.
Californium(III) chloride is an inorganic compound with a chemical formula CfCl3. Like in californium oxide (Cf2O3) and other californium halides, including californium fluoride (CfF3) and iodide (CfI3), the californium atom has an oxidation state of +3.

Californium(III) bromide is an inorganic compound, a salt with a chemical formula CfBr3. Like in californium oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3), californium(III) chloride, and californium(III) iodide (CfI3), the californium atom has an oxidation state of +3.

Berkelium(IV) oxide, also known as berkelium dioxide, is a chemical compound with the formula BkO2. This compound slowly decays to californium(IV) oxide. It can be converted to berkelium(III) oxide by hydrogen reduction at 600 °C.
Curium compounds are compounds containing the element curium (Cm). Curium usually forms compounds in the +3 oxidation state, although compounds with curium in the +4, +5 and +6 oxidation states are also known.

Berkelium(III) nitrate is the berkelium salt of nitric acid with the formula Bk(NO3)3. It commonly forms the tetrahydrate, Bk(NO3)3·4H2O, which is a light green solid. If heated to 450 °C, it decomposes to berkelium(IV) oxide and 22 milligrams of the solution of this compound is reported to cost one million dollars.

Berkelium(III) chloride also known as berkelium trichloride, is a chemical compound with the formula BkCl3. It is a water-soluble green salt with a melting point of 603 °C. This compound forms the hexahydrate, BkCl3·6H2O.
Protactinium compounds are compounds containing the element protactinium. These compounds usually have protactinium in the +5 oxidation state, although these compounds can also exist in the +2, +3 and +4 oxidation states.
Americium compounds are compounds containing the element americium (Am). These compounds can form in the +2, +3, and +4, although the +3 oxidation state is the most common. The +5, +6 and +7 oxidation states have also been reported.

Berkelium(III) fluoride is a binary inorganic compound of berkelium and fluorine with the chemical formula BkF
3.

Berkelium bromide is a bromide of berkelium, with the chemical formula BkBr3.
Einsteinium fluoride is a binary inorganic chemical compound of einsteinium and fluorine with the chemical formula EsF3.













