Related Research Articles

The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together with hydrogen they constitute group 1, which lies in the s-block of the periodic table. All alkali metals have their outermost electron in an s-orbital: this shared electron configuration results in their having very similar characteristic properties. Indeed, the alkali metals provide the best example of group trends in properties in the periodic table, with elements exhibiting well-characterised homologous behaviour. This family of elements is also known as the lithium family after its leading element.

Caesium is a chemical element; it has symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of 28.5 °C (83.3 °F), which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at −116 °C (−177 °F). It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometers.
Francium is a chemical element; it has symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223, has a half-life of only 22 minutes. It is the second-most electropositive element, behind only caesium, and is the second rarest naturally occurring element. Francium's isotopes decay quickly into astatine, radium, and radon. The electronic structure of a francium atom is [Rn] 7s1; thus, the element is classed as an alkali metal.

Radium is a chemical element; it has symbol Ra and atomic number 88. It is the sixth element in group 2 of the periodic table, also known as the alkaline earth metals. Pure radium is silvery-white, but it readily reacts with nitrogen (rather than oxygen) upon exposure to air, forming a black surface layer of radium nitride (Ra3N2). All isotopes of radium are radioactive, the most stable isotope being radium-226 with a half-life of 1,600 years. When radium decays, it emits ionizing radiation as a by-product, which can excite fluorescent chemicals and cause radioluminescence.

In chemistry, a salt or ionic compound is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a neutral compound with no net electric charge. The constituent ions are held together by electrostatic forces termed ionic bonds.

Ununennium, also known as eka-francium or element 119, is a hypothetical chemical element; it has symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be an s-block element, an alkali metal, and the first element in the eighth period. It is the lightest element that has not yet been synthesized.

Caesium fluoride or cesium fluoride is an inorganic compound with the formula CsF and it is a hygroscopic white salt. Caesium fluoride can be used in organic synthesis as a source of the fluoride anion. Caesium also has the highest electropositivity of all known elements and fluorine has the highest electronegativity of all known elements.
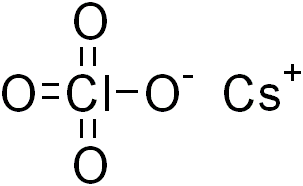
Caesium perchlorate or cesium perchlorate (CsClO4), is a perchlorate of caesium. It forms white crystals, which are sparingly soluble in cold water and ethanol. It dissolves more easily in hot water.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.
Iodine compounds are compounds containing the element iodine. Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.

Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.
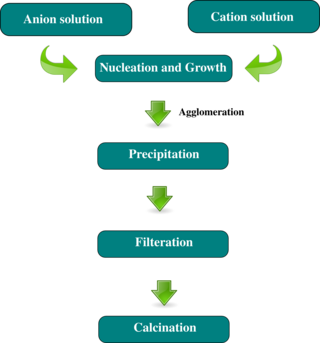
In chemistry, coprecipitation (CPT) or co-precipitation is the carrying down by a precipitate of substances normally soluble under the conditions employed. Analogously, in medicine, coprecipitation is specifically "an assay designed to purify a single antigen from a complex mixture using a specific antibody attached to a beaded support".
The alkali hydroxides are a class of chemical compounds which are composed of an alkali metal cation and the hydroxide anion. The alkali hydroxides are:
Polonium trioxide (also known as polonium(VI) oxide) is a chemical compound with the formula PoO3. It is one of three oxides of polonium, the other two being polonium monoxide (PoO) and polonium dioxide (PoO2). It is an interchalcogen that has so far only been detected in trace amounts.
Perchloratoborate is an anion of the form [B(ClO4)4]−. It can form partly stable solid salts with heavy alkali metals. They are more stable than nitratoborate salts. K[B(ClO4)4] decomposes at 35 °C, Rb[B(ClO4)4] is stable to 50 °C, and Cs[B(ClO4)4] can exist up to 80 °C.

Platinum tetrafluoride is the inorganic compound with the chemical formula PtF
4. In the solid state, the compound features platinum(IV) in octahedral coordination geometry.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Francium chloride is a radioactive chemical compound with the formula FrCl. It is a salt predicted to be a white solid and is soluble in water. Its properties resemble caesium chloride.

Astatine compounds are compounds that contain the element astatine (At). As this element is very radioactive, few compounds have been studied. Less reactive than iodine, astatine is the least reactive of the halogens. Its compounds have been synthesized in nano-scale amounts and studied as intensively as possible before their radioactive disintegration. The reactions involved have been typically tested with dilute solutions of astatine mixed with larger amounts of iodine. Acting as a carrier, the iodine ensures there is sufficient material for laboratory techniques to work. Like iodine, astatine has been shown to adopt odd-numbered oxidation states ranging from −1 to +7.
Radium compounds are compounds containing the element radium (Ra). Due to radium's radioactivity, not many compounds have been well characterized. Solid radium compounds are white as radium ions provide no specific coloring, but they gradually turn yellow and then dark over time due to self-radiolysis from radium's alpha decay. Insoluble radium compounds coprecipitate with all barium, most strontium, and most lead compounds.
References
- ↑ Hyde, E. K. (1952). "Radiochemical Methods for the Isolation of Element 87 (Francium)". J. Am. Chem. Soc. 74 (16): 4181–4184. doi:10.1021/ja01136a066. hdl: 2027/mdp.39015086483156 . S2CID 95854270.
- 1 2 3 E. N K. Hyde Radiochemistry of Francium, Subcommittee on Radiochemistry, National Academy of Sciences-National Research Council; available from the Office of Technical Services, Dept. of Commerce, 1960.
- 1 2 3 Lavrukhina, Avgusta Konstantinovna; Pozdnyakov, Aleksandr Aleksandrovich (1970). Analytical Chemistry of Technetium, Promethium, Astatine, and Francium. Translated by R. Kondor. Ann Arbor–Humphrey Science Publishers. p. 269. ISBN 978-0-250-39923-9.
- ↑ Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements". Relativistic Methods for Chemists. Challenges and Advances in Computational Chemistry and Physics. Vol. 10. Springer. p. 81. doi:10.1007/978-1-4020-9975-5_2. ISBN 978-1-4020-9975-5.
- ↑ Cao, Chang-Su; Hu, Han-Shi; Schwarz, W. H. Eugen; Li, Jun (2022). "Periodic Law of Chemistry Overturns for Superheavy Elements". ChemRxiv (preprint). doi:10.26434/chemrxiv-2022-l798p . Retrieved 16 November 2022.