
Bismuth forms mainly trivalent and a few pentavalent compounds. Many of its chemical properties are similar to those of arsenic and antimony, although much less toxic. [1]

Bismuth forms mainly trivalent and a few pentavalent compounds. Many of its chemical properties are similar to those of arsenic and antimony, although much less toxic. [1]
At elevated temperatures, vaporized bismuth metal and oxygen combine into the yellow trioxide, Bi
2O
3. [2] [3] At temperatures above 710 °C, this (molten) oxide corrodes all known oxides and even platinum. [4] It forms two series of oxyanions in basic conditions: linear, chain-polymeric BiO−
2; and cubic BiO3−
3. In Li
3BiO
3, the anion forms the octamer Bi
8O24−
24; in Na
3BiO
3, the tetramer. [5]
The dark red bismuth(V) oxide, Bi
2O
5, is unstable, liberating O
2 gas upon heating. [6] The compound NaBiO3 is a strong oxidant. [7]
Bismuth sulfide, Bi
2S
3, occurs naturally in bismuth ores, [8] but can be synthesized from molten bismuth and sulfur. [9]
In oxidation state +3, bismuth forms salts with all the halogens: BiF
3, BiCl
3, BiBr
3, and BiI
3. All hydrolyze in water except BiF
3. [5] Bismuth(III) chloride reacts with hydrogen chloride in ether solution to produce the acid HBiCl
4. [10]
The oxidation state +5 is less frequently encountered. One such compound is the powerful oxidant and fluorinator, BiF
5. It is also a strong fluoride acceptor, forming the XeF+
3 cation from xenon tetrafluoride: [10]
The low-oxidation-state bismuth halides adopt unusual cluster structures. What was originally thought bismuth(I) chloride, BiCl, is in fact a lattice of Bi5+
9 cations and BiCl2−
5 and Bi
2Cl2−
8 anions. [5] [11] The Bi5+
9 cation has a distorted tricapped trigonal prismatic molecular geometry and is also found in Bi
10Hf
3Cl
18, which is prepared by reducing a mixture of hafnium(IV) chloride and bismuth chloride with elemental bismuth, having the structure [Bi+
] [Bi5+
9] [HfCl2−
6]
3. [5] : 50 Other polyatomic bismuth cations are also known, such as Bi2+
8, found in Bi
8(AlCl
4)
2. [11]
There is a true monoiodide, BiI, which contains chains of Bi
4I
4 units. BiI decomposes upon heating to the triiodide, BiI
3, and elemental bismuth. [5]
Bismuth forms at least two "monobromides": one isostructural to "BiCl"[ citation needed ] and one isostructural to Bi
4I
4. [5]
In aqueous solution, the Bi3+
ion is solvated to form the aqua ion Bi(H
2O)3+
8 in strongly acidic conditions. [12] At pH > 0 polynuclear species exist, the most important of which is believed to be the octahedral complex [Bi
6O
4(OH)
4]6+
. [13]

Bismuth oxychloride (BiOCl) and bismuth oxynitrate (BiONO3) stoichiometrically appear simple anionic salts of the bismuthyl(III) cation (BiO+), which commonly occurs in aqueous bismuth compounds. However, in the case of BiOCl, the salt crystal forms alternating plates of Bi, O, and Cl atoms. Each oxygen coordinates with four bismuth atoms in the adjacent plane. [14]
Unlike the lighter pnictogens nitrogen, phosphorus, and arsenic, but similar to antimony, bismuth does not form a stable hydride. Bismuth hydride, bismuthine (BiH
3), is an endothermic compound that spontaneously decomposes at room temperature. It is stable only below −60 °C. [5] Bismuthides are intermetallic compounds between bismuth and other metals. [15]
In 2014 researchers discovered that sodium bismuthide admits bulk 3D Dirac fermions. As a topological Dirac semi-metal, it is a three-dimensional counterpart to graphene with similar electron mobility and velocity. While sodium bismuthide (Na
3Bi) is too unstable to be used in devices without packaging, it may offer distinct efficiency and fabrication advantages over planar graphene in semiconductor and spintronics applications. [16] [17]


Antimony is a chemical element; it has symbol Sb (from Latin stibium) and atomic number 51. A lustrous gray metalloid, it is found in nature mainly as the sulfide mineral stibnite (Sb2S3). Antimony compounds have been known since ancient times and were powdered for use as medicine and cosmetics, often known by the Arabic name kohl. The earliest known description of the metalloid in the West was written in 1540 by Vannoccio Biringuccio.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
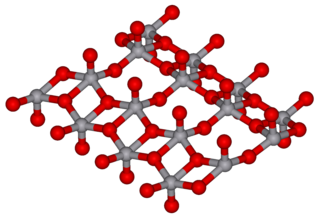
Vanadium(V) oxide (vanadia) is the inorganic compound with the formula V2O5. Commonly known as vanadium pentoxide, it is a brown/yellow solid, although when freshly precipitated from aqueous solution, its colour is deep orange. Because of its high oxidation state, it is both an amphoteric oxide and an oxidizing agent. From the industrial perspective, it is the most important compound of vanadium, being the principal precursor to alloys of vanadium and is a widely used industrial catalyst.

Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

Ammonium dichromate is an inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a salt consisting of ammonium ions and dichromate ions.

Bismuth chloride (or butter of bismuth) is an inorganic compound with the chemical formula BiCl3. It is a covalent compound and is the common source of the Bi3+ ion. In the gas phase and in the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory.

Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery-white color when freshly produced. Surface oxidation generally gives samples of the metal a somewhat rosy cast. Further oxidation under heat can give bismuth a vividly iridescent appearance due to thin-film interference. Bismuth is both the most diamagnetic element and one of the least thermally conductive metals known.

Bismuth oxychloride is an inorganic compound of bismuth with the formula BiOCl. It is a lustrous white solid used since antiquity, notably in ancient Egypt. Light wave interference from its plate-like structure gives a pearly iridescent light reflectivity similar to nacre. It is also known as pearl white.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.

Bismuth vanadate is the inorganic compound with the formula BiVO4. It is a bright yellow solid. It is widely studied as visible light photo-catalyst with a narrow band gap of less than 2.4 eV. It is a representative of "complex inorganic colored pigments," or CICPs. More specifically, bismuth vanadate is a mixed-metal oxide. Bismuth vanadate is also known under the Colour Index International as C.I. Pigment Yellow 184. It occurs naturally as the rare minerals pucherite, clinobisvanite, and dreyerite.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
Gallium compounds are compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2. There are also compounds of gallium with negative oxidation states, ranging from -5 to -1, most of these compounds being magnesium gallides (MgxGay).

Praseodymium bismuthide is a binary inorganic compound of praseodymium and bismuth with the chemical formula of PrBi. It forms crystals.

Bismuthyl — inorganic oxygen-containing singly charged ion with the chemical formula BiO+, is an oxycation of bismuth in the +3 oxidation state. Most often it is formed during the hydrolysis of trivalent bismuth salts, primarily nitrate, chloride and other halides. In chemical compounds, bismuthyl plays the role of a monovalent cation.