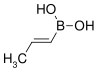
A boronic acid is an organic compound related to boric acid (B(OH)3) in which one of the three hydroxyl groups (−OH) is replaced by an alkyl or aryl group (represented by R in the general formula R−B(OH)2). [1] As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes.
Contents
- Structure and synthesis
- Synthesis
- Boronic esters (also named boronate esters)
- Organic chemistry applications
- Suzuki coupling reaction
- Chan–Lam coupling
- Liebeskind–Srogl coupling
- Conjugate addition
- Oxidation
- Homologation
- Electrophilic allyl shifts
- Hydrolysis
- C–H coupling reactions
- Protonolysis
- Supramolecular chemistry
- Saccharide recognition
- Safety
- Notes
- References
- External links
Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc. (molecules with vicinal, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol, amine, carboxylate)). The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the Suzuki coupling. A key concept in its chemistry is transmetallation of its organic residue to a transition metal.
The compound bortezomib with a boronic acid group is a drug used in chemotherapy. The boron atom in this molecule is a key substructure because through it certain proteasomes are blocked that would otherwise degrade proteins. Boronic acids are known to bind to active site serines and are part of inhibitors for porcine pancreatic lipase, [2] subtilisin [3] and the protease Kex2. [4] Furthermore, boronic acid derivatives constitute a class of inhibitors for human acyl-protein thioesterase 1 and 2, which are cancer drug targets within the Ras cycle. [5]