
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n−2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

Organoboron chemistry or organoborane chemistry studies organoboron compounds, also called organoboranes. These chemical compounds combine boron and carbon; typically, they are organic derivatives of borane (BH3), as in the trialkyl boranes.

9-Borabicyclo[3.3.1]nonane or 9-BBN is an organoborane compound. This colourless solid is used in organic chemistry as a hydroboration reagent. The compound exists as a hydride-bridged dimer, which easily cleaves in the presence of reducible substrates. 9-BBN is also known by its nickname 'banana borane'. This is because rather than drawing out the full structure, chemists often simply draw a banana shape with the bridging boron.

Palladium(II) acetate is a chemical compound of palladium described by the formula [Pd(O2CCH3)2]n, abbreviated [Pd(OAc)2]n. It is more reactive than the analogous platinum compound. Depending on the value of n, the compound is soluble in many organic solvents and is commonly used as a catalyst for organic reactions.

Diglyme, or bis(2-methoxyethyl) ether, is an organic compound with the chemical formula (CH3OCH2CH2)2O. It is a colorless liquid with a slight ether-like odor. It is a solvent with a high boiling point. It is the dimethyl ether of diethylene glycol. The name diglyme is a portmanteau of diglycol methyl ether. It is miscible with water as well as organic solvents.

Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.

Disiamylborane is an organoborane with the formula [( 2CHCH )2BH]2. It is a colorless waxy solid that is used in organic synthesis for hydroboration–oxidation reactions. Like most dialkyl boron hydrides, it has a dimeric structure with bridging hydrides.

Borane dimethylsulfide (BMS) is a chemical compound with the chemical formula BH3·S(CH3)2. It is an adduct between borane molecule and dimethyl sulfide molecule. It is a complexed borane reagent that is used for hydroborations and reductions. The advantages of BMS over other borane reagents, such as borane-tetrahydrofuran, are its increased stability and higher solubility. BMS is commercially available at much higher concentrations than its tetrahydrofuran counterpart and does not require sodium borohydride as a stabilizer, which could result in undesired side reactions. In contrast, BH3·THF requires sodium borohydride to inhibit reduction of THF to tributyl borate. BMS is soluble in most aprotic solvents.

Sodium triacetoxyborohydride, also known as sodium triacetoxyhydroborate, commonly abbreviated STAB, is a chemical compound with the formula Na[(CH3COO)3BH]. Like other borohydrides, it is used as a reducing agent in organic synthesis. This colourless salt is prepared by protonolysis of sodium borohydride with acetic acid:
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Boronic acids and esters are classified depending on the type of carbon group (R) directly bonded to boron, for example alkyl-, alkenyl-, alkynyl-, and aryl-boronic esters. The most common type of starting materials that incorporate boronic esters into organic compounds for transition metal catalyzed borylation reactions have the general formula (RO)2B-B(OR)2. For example, bis(pinacolato)diboron (B2Pin2), and bis(catecholato)diborane (B2Cat2) are common boron sources of this general formula.
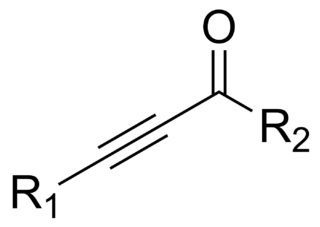
In organic chemistry, an ynone is an organic compound containing a ketone functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.
In organic chemistry, methylenation is a chemical reaction that inserts a methylene group into a chemical compound:

(Trimethylsilyl)methyllithium is classified both as an organolithium compound and an organosilicon compound. It has the empirical formula LiCH2Si(CH3)3, often abbreviated LiCH2tms. It crystallizes as the hexagonal prismatic hexamer [LiCH2tms]6, akin to some polymorphs of methyllithium. Many adducts have been characterized including the diethyl ether complexed cubane [Li4(μ3-CH2tms)4(Et2O)2] and [Li2(μ-CH2tms)2(tmeda)2].



















