Food physical chemistry is considered to be a branch of food chemistry [1] [2] concerned with the study of both physical and chemical interactions in foods in terms of physical and chemical principles applied to food systems, as well as the applications of physical/chemical techniques and instrumentation for the study of foods. [3] [4] [5] [6] This field encompasses the "physiochemical principles of the reactions and conversions that occur during the manufacture, handling, and storage of foods." [7]
Contents
- Topics in Food physical chemistry
- Related fields
- Techniques gallery: High-Field NMR, CARS (Raman spectroscopy), Fluorescence confocal microscopy and Hyperspectral imaging
- See also
- References
- Journals
- External links
Food physical chemistry concepts are often drawn from rheology, theories of transport phenomena, physical and chemical thermodynamics, chemical bonds and interaction forces, quantum mechanics and reaction kinetics, biopolymer science, colloidal interactions, nucleation, glass transitions, and freezing, [8] [9] disordered/noncrystalline solids.
| | This section needs expansionwith: actual complete English sentence containing relevance to food physical chemistry. You can help by adding to it. (January 2022) |
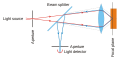
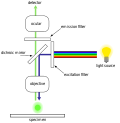
Techniques utilized range widely from dynamic rheometry, optical microscopy, electron microscopy, AFM, light scattering, X-ray diffraction/neutron diffraction, [10] to MRI, spectroscopy (NMR, [11] FT-NIR/IR, NIRS, ESR and EPR, [12] [13] CD/VCD, [14] Fluorescence, FCS, [15] [16] [17] [18] [19] HPLC, GC-MS, [20] [21] and other related analytical techniques.
Understanding food processes and the properties of foods requires a knowledge of physical chemistry and how it applies to specific foods and food processes. Food physical chemistry is essential for improving the quality of foods, their stability, and food product development. Because food science is a multi-disciplinary field, food physical chemistry is being developed through interactions with other areas of food chemistry and food science, such as food analytical chemistry, food process engineering/food processing, food and bioprocess technology, food extrusion, food quality control, food packaging, food biotechnology, and food microbiology.