
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized.

Photoluminescence is light emission from any form of matter after the absorption of photons. It is one of many forms of luminescence and is initiated by photoexcitation, hence the prefix photo-. Following excitation, various relaxation processes typically occur in which other photons are re-radiated. Time periods between absorption and emission may vary: ranging from short femtosecond-regime for emission involving free-carrier plasma in inorganic semiconductors up to milliseconds for phosphoresence processes in molecular systems; and under special circumstances delay of emission may even span to minutes or hours.

An excimer is a short-lived dimeric or heterodimeric molecule formed from two species, at least one of which has a valence shell completely filled with electrons. In this case, formation of molecules is possible only if such atom is in an electronic excited state. Heteronuclear molecules and molecules that have more than two species are also called exciplex molecules. Excimers are often diatomic and are composed of two atoms or molecules that would not bond if both were in the ground state. The lifetime of an excimer is very short, on the order of nanoseconds. Binding of a larger number of excited atoms forms Rydberg matter clusters, the lifetime of which can exceed many seconds.

Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet, visible light (400–750 nm) or infrared radiation (750–2500 nm).

In quantum mechanics, an excited state of a system is any quantum state of the system that has a higher energy than the ground state. Excitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being raised to an excited state. The temperature of a group of particles is indicative of the level of excitation.
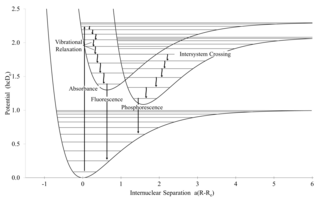
Intersystem crossing (ISC) is an isoenergetic radiationless process involving a transition between the two electronic states with different states spin multiplicity.
In physics and physical chemistry, time-resolved spectroscopy is the study of dynamic processes in materials or chemical compounds by means of spectroscopic techniques. Most often, processes are studied after the illumination of a material occurs, but in principle, the technique can be applied to any process that leads to a change in properties of a material. With the help of pulsed lasers, it is possible to study processes that occur on time scales as short as 10−16 seconds.

In the process of photosynthesis, the phosphorylation of ADP to form ATP using the energy of sunlight is called photophosphorylation. Cyclic photophosphorylation occurs in both aerobic and anaerobic conditions. Only two sources of energy are available to living organisms: sunlight and reduction-oxidation (redox) reactions. All organisms produce ATP, which is the universal energy currency of life. In photosynthesis this commonly involves photolysis, or photodissociation, of water and a continuous unidirectional flow of electrons from water to photosystem II.

A dye-sensitized solar cell is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a photoelectrochemical system. The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O'Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne (EPFL) until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention.

Photosensitizers produce a physicochemical change in a neighboring molecule by either donating an electron to the substrate or by abstracting a hydrogen atom from the substrate. At the end of this process, the photosensitizer eventually returns to its ground state, where it remains chemically intact until the photosensitizer absorbs more light. This means that the photosensitizer remains unchanged before and after the energetic exchange, much like heterogeneous photocatalysis. One branch of chemistry which frequently utilizes photosensitizers is polymer chemistry, using photosensitizers in reactions such as photopolymerization, photocrosslinking, and photodegradation. Photosensitizers are also used to generate prolonged excited electronic states in organic molecules with uses in photocatalysis, photon upconversion and photodynamic therapy. Generally, photosensitizers absorb electromagnetic radiation consisting of infrared radiation, visible light radiation, and ultraviolet radiation and transfer absorbed energy into neighboring molecules. This absorption of light is made possible by photosensitizers' large de-localized π-systems, which lowers the energy of HOMO and LUMO orbitals to promote photoexcitation. While many photosensitizers are organic or organometallic compounds, there are also examples of using semiconductor quantum dots as photosensitizers.
Photodissociation, photolysis, or photodecomposition is a chemical reaction in which a chemical compound is broken down by photons. It is defined as the interaction of one or more photons with one target molecule. Photodissociation is not limited to visible light. Any photon with sufficient energy can affect the chemical bonds of a chemical compound. Since a photon's energy is inversely proportional to its wavelength, electromagnetic waves with the energy of visible light or higher, such as ultraviolet light, x-rays and gamma rays are usually involved in such reactions.
Hybrid solar cells combine advantages of both organic and inorganic semiconductors. Hybrid photovoltaics have organic materials that consist of conjugated polymers that absorb light as the donor and transport holes. Inorganic materials in hybrid cells are used as the acceptor and electron transporter in the structure. The hybrid photovoltaic devices have a potential for not only low-cost by roll-to-roll processing but also for scalable solar power conversion.

A photosynthetic reaction center is a complex of several proteins, pigments and other co-factors that together execute the primary energy conversion reactions of photosynthesis. Molecular excitations, either originating directly from sunlight or transferred as excitation energy via light-harvesting antenna systems, give rise to electron transfer reactions along the path of a series of protein-bound co-factors. These co-factors are light-absorbing molecules such as chlorophyll and phaeophytin, as well as quinones. The energy of the photon is used to excite an electron of a pigment. The free energy created is then used to reduce a chain of nearby electron acceptors, which have progressively higher redox-potentials. These electron transfer steps are the initial phase of a series of energy conversion reactions, ultimately resulting in the conversion of the energy of photons to the storage of that energy by the production of chemical bonds.
A photochemical logic gate is based on the photochemical intersystem crossing and molecular electronic transition between photochemically active molecules, leading to logic gates that can be produced.

In photosynthesis, the light-dependent reactions take place on the thylakoid membranes. The inside of the thylakoid membrane is called the lumen, and outside the thylakoid membrane is the stroma, where the light-independent reactions take place. The thylakoid membrane contains some integral membrane protein complexes that catalyze the light reactions. There are four major protein complexes in the thylakoid membrane: Photosystem II (PSII), Cytochrome b6f complex, Photosystem I (PSI), and ATP synthase. These four complexes work together to ultimately create the products ATP and NADPH.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
Transition metal oxides are compounds composed of oxygen atoms bound to transition metals. They are commonly utilized for their catalytic activity and semiconductive properties. Transition metal oxides are also frequently used as pigments in paints and plastics, most notably titanium dioxide. Transition metal oxides have a wide variety of surface structures which affect the surface energy of these compounds and influence their chemical properties. The relative acidity and basicity of the atoms present on the surface of metal oxides are also affected by the coordination of the metal cation and oxygen anion, which alter the catalytic properties of these compounds. For this reason, structural defects in transition metal oxides greatly influence their catalytic properties. The acidic and basic sites on the surface of metal oxides are commonly characterized via infrared spectroscopy, calorimetry among other techniques. Transition metal oxides can also undergo photo-assisted adsorption and desorption that alter their electrical conductivity. One of the more researched properties of these compounds is their response to electromagnetic radiation, which makes them useful catalysts for redox reactions, isotope exchange and specialized surfaces.
An excimer lamp is a source of ultraviolet light produced by spontaneous emission of excimer (exciplex) molecules.
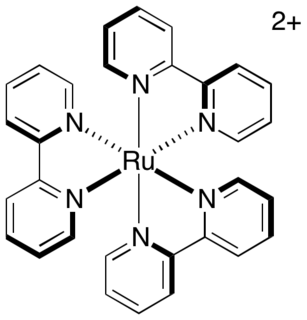
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
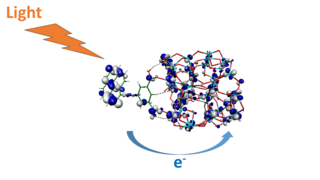
Quantum photoelectrochemistry is the investigation of the quantum mechanical nature of photoelectrochemistry, the subfield of study within physical chemistry concerned with the interaction of light with electrochemical systems, typically through the application of quantum chemical calculations. Quantum photoelectrochemistry provides an expansion of quantum electrochemistry to processes involving also the interaction with light (photons). It therefore also includes essential elements of photochemistry. Key aspects of quantum photoelectrochemistry are calculations of optical excitations, photoinduced electron and energy transfer processes, excited state evolution, as well as interfacial charge separation and charge transport in nanoscale energy conversion systems.










