Lead sulfide refers to two compounds containing lead and sulfur:
- Lead(II) sulfide, PbS, containing lead in the +2 oxidation state, naturally occurring as the mineral galena
- Lead(IV) sulfide, PbS2, containing lead in the +4 oxidation state
Lead sulfide refers to two compounds containing lead and sulfur:

Lead is a chemical element with the symbol Pb and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children.
Sulfide (British English also sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. Sulfide also refers to chemical compounds large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (SH−) are the conjugate acids of sulfide.

Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.

Lead glass, commonly called crystal, is a variety of glass in which lead replaces the calcium content of a typical potash glass. Lead glass contains typically 18–40% lead(II) oxide (PbO), while modern lead crystal, historically also known as flint glass due to the original silica source, contains a minimum of 24% PbO. Lead glass is often desirable for a variety of uses due to its clarity. In marketing terms it is often called crystal glass.

Lead(II) sulfide is an inorganic compound with the formula PbS. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses.

Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula PbO. PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. Modern applications for PbO are mostly in lead-based industrial glass and industrial ceramics, including computer components. It is an amphoteric oxide.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.

Lead(II,IV) oxide, also called red lead or minium, is the inorganic compound with the formula . A bright red or orange solid, it is used as pigment, in the manufacture of batteries, and rustproof primer paints. It is an example of a mixed valence compound, being composed of both Pb(II) and Pb(IV) in the ratio of two to one.

Lead(II) acetate, also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, or Goulard's powder, is a white crystalline chemical compound with a slightly sweet taste. Its chemical formula is usually expressed as Pb(CH3COO)2 or Pb(OAc)2, where Ac represents the acetyl group. Like many other lead compounds, it is toxic. Lead acetate is soluble in water and glycerin. With water it forms the trihydrate, Pb(OAc)2·3H2O, a colourless or white efflorescent monoclinic crystalline substance.

Lead(IV) oxide, commonly known as lead dioxide, is the inorganic compound with the formula PbO2. It is an oxide where lead is in an oxidation state of +4. It is a dark-brown solid which is insoluble in water. It exists in two crystalline forms. It has several important applications in electrochemistry, in particular as the positive plate of lead acid batteries.
Lead(II) hydroxide, Pb(OH)2, is a hydroxide of lead, with lead in oxidation state +2. In 1964 it was believed that such a simple compound did not exist, as lead basic carbonate (2PbCO3·Pb(OH)2) or lead(II) oxide (PbO) was encountered where lead hydroxide was expected. This has been a subject of considerable confusion in the past. However, subsequent research has demonstrated that lead(II) hydroxide does indeed exist as one of a series of lead hydroxides.
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen (O).

Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.
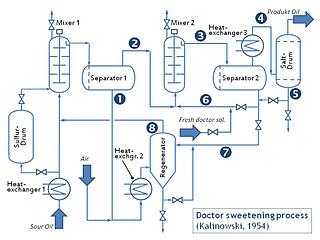
The doctor sweetening process is an industrial chemical process for converting mercaptans in sour gasoline into disulfides. Sulfur compounds darken gasoline, give it an offensive odor and increase toxic sulfur dioxide engine emissions. However, this process only reduces the odor.
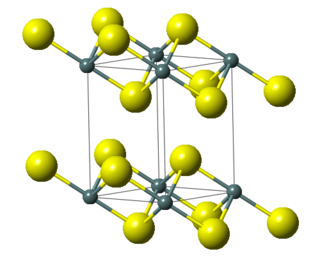
Lead(IV) sulfide is a chemical compound with the formula PbS2. This material is generated by the reaction of the more common lead(II) sulfide, PbS, with sulfur at >600 °C and at high pressures. PbS2, like the related tin(IV) sulfide SnS2, crystallises in the cadmium iodide motif, which indicates that Pb should be assigned the formal oxidation state of 4+.

Lead(II) bromide is the inorganic compound with the formula PbBr2. It is a white powder. It is produced in the burning of typical leaded gasolines.

Compounds of lead exist with lead in two main oxidation states: +2 and +4. The former is more common. Inorganic lead(IV) compounds are typically strong oxidants or exist only in highly acidic solutions.