A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3.

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.

In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the corners are identical, the molecule belongs to point group C3v. Some molecules and ions with trigonal pyramidal geometry are the pnictogen hydrides (XH3), xenon trioxide (XeO3), the chlorate ion, ClO−
3, and the sulfite ion, SO2−
3. In organic chemistry, molecules which have a trigonal pyramidal geometry are sometimes described as sp3 hybridized. The AXE method for VSEPR theory states that the classification is AX3E1.

In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to the point group D3h. Molecules where the three ligands are not identical, such as H2CO, deviate from this idealized geometry. Examples of molecules with trigonal planar geometry include boron trifluoride (BF3), formaldehyde (H2CO), phosgene (COCl2), and sulfur trioxide (SO3). Some ions with trigonal planar geometry include nitrate (NO−
3), carbonate (CO2−
3), and guanidinium (C(NH
2)+
3). In organic chemistry, planar, three-connected carbon centers that are trigonal planar are often described as having sp2 hybridization.
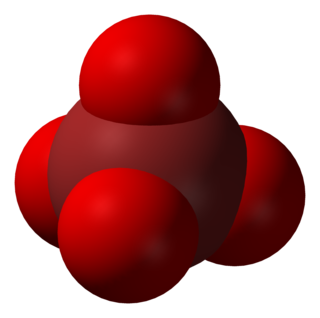
In chemistry, the perbromate ion is the anion having the chemical formula BrO−
4. It is an oxyanion of bromine, the conjugate base of perbromic acid, in which bromine has the oxidation state +7. Unlike its chlorine and iodine analogs, it is difficult to synthesize. It has tetrahedral molecular geometry.
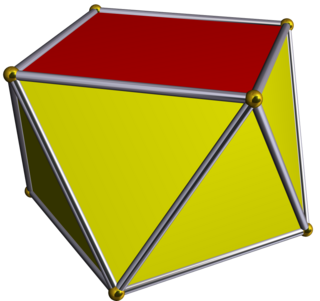
In geometry, the square antiprism is the second in an infinite family of antiprisms formed by an even-numbered sequence of triangle sides closed by two polygon caps. It is also known as an anticube.

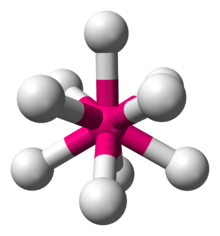
Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group D5h.

Potassium nonahydridorhenate(VII) is an inorganic compound having the formula K2[ReH9]. This colourless salt is soluble in water but only poorly soluble in most alcohols. This salt contains the nonahydridorhenate(VII) anion, [ReH9]2−, which is a rare example of a coordination complex bearing only hydride ligands.
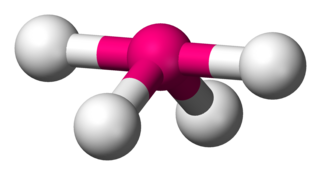
Disphenoidal or seesaw is a type of molecular geometry where there are four bonds to a central atom with overall C2v molecular symmetry. The name "seesaw" comes from the observation that it looks like a playground seesaw. Most commonly, four bonds to a central atom result in tetrahedral or, less commonly, square planar geometry.

The linear molecular geometry describes the geometry around a central atom bonded to two other atoms placed at a bond angle of 180°. Linear organic molecules, such as acetylene, are often described by invoking sp orbital hybridization for their carbon centers.
Ammonium fluorosilicate (also known as ammonium hexafluorosilicate, ammonium fluosilicate or ammonium silicofluoride) has the formula (NH4)2SiF6. It is a toxic chemical, like all salts of fluorosilicic acid. It is made of white crystals, which have at least three polymorphs and appears in nature as rare minerals cryptohalite or bararite.
Polysilicon halides are silicon-backbone polymeric solids. At room temperature, the polysilicon fluorides are colorless to yellow solids while the chlorides, bromides, and iodides are, respectively, yellow, amber, and red-orange. Polysilicon dihalides (perhalo-polysilenes) have the general formula (SiX2)n while the polysilicon monohalides (perhalo-polysilynes) have the formula (SiX)n, where X is F, Cl, Br, or I and n is the number of monomer units in the polymer.

Disulfur dioxide, dimeric sulfur monoxide or SO dimer is an oxide of sulfur with the formula S2O2. The solid is unstable with a lifetime of a few seconds at room temperature.
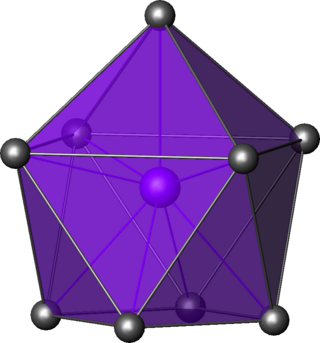
In chemistry, the capped square antiprismatic molecular geometry describes the shape of compounds where nine atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a gyroelongated square pyramid.
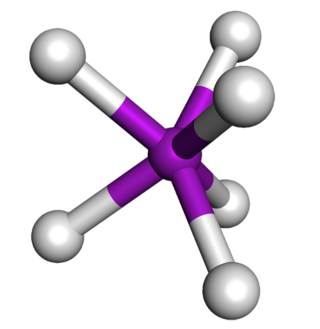
In chemistry, the trigonal prismatic molecular geometry describes the shape of compounds where six atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a triangular prism.

In chemistry, the capped octahedral molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a gyroelongated triangular pyramid. This shape has C3v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped trigonal prism.

In chemistry, the capped trigonal prismatic molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of an augmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped octahedron.

In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid. This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism.

In chemistry, the bicapped trigonal prismatic molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a biaugmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the dodecahedron.