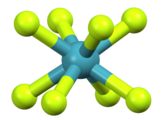
| Square antiprismatic molecular geometry | |
|---|---|
 | |
| Examples | XeF2− 8, ReF− 8 |
| Point group | D4d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism. [1] This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism. [2] [3]
Contents
Like with other high coordination numbers, eight-coordinate compounds are often distorted from idealized geometries, as illustrated by the structure of Na3TaF8. In this case, with the small Na+ ions, lattice forces are strong. With the diatomic cation NO+, the lattice forces are weaker, such as in (NO)2XeF8, which crystallizes with a more idealized square antiprismatic geometry.
- The distorted square antiprismatic [TaF8]3− anion in the Na3TaF8 lattice. [4]
- The square antiprismatic [XeF8]2− anion in the lattice of nitrosonium octafluoroxenate(VI), (NO)2XeF8. [5]
- Structure of the Bi2+
8 cluster in the [Bi8](GaCl4)2.