
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.

Valence shell electron pair repulsion (VSEPR) theory, is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.

In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to the point group D3h. Molecules where the three ligands are not identical, such as H2CO, deviate from this idealized geometry. Examples of molecules with trigonal planar geometry include boron trifluoride (BF3), formaldehyde (H2CO), phosgene (COCl2), and sulfur trioxide (SO3). Some ions with trigonal planar geometry include nitrate (NO−
3), carbonate (CO2−
3), and guanidinium (C(NH
2)+
3). In organic chemistry, planar, three-connected carbon centers that are trigonal planar are often described as having sp2 hybridization.

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.

Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI.
In chemistry, crystallography, and materials science, the coordination number, also called ligancy, of a central atom in a molecule or crystal is the number of atoms, molecules or ions bonded to it. The ion/molecule/atom surrounding the central ion/molecule/atom is called a ligand. This number is determined somewhat differently for molecules than for crystals.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group D5h.
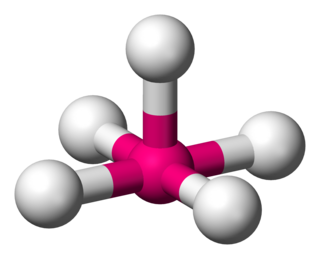
Square pyramidal geometry describes the shape of certain chemical compounds with the formula ML5 where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably [Ni(CN)5]3−.
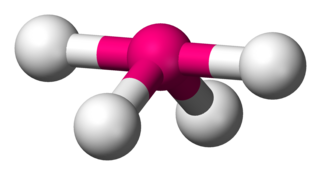
Disphenoidal or seesaw is a type of molecular geometry where there are four bonds to a central atom with overall C2v molecular symmetry. The name "seesaw" comes from the observation that it looks like a playground seesaw. Most commonly, four bonds to a central atom result in tetrahedral or, less commonly, square planar geometry.
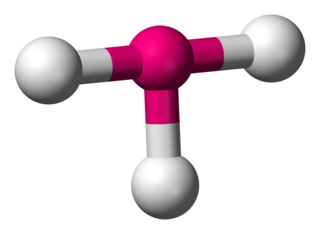
In chemistry, T-shaped molecular geometry describes the structures of some molecules where a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. Examples of T-shaped molecules are the halogen trifluorides, such as ClF3.
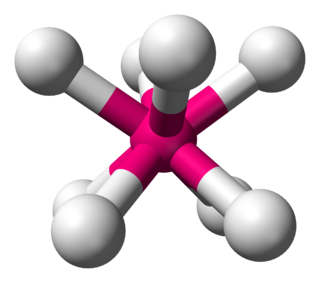
In chemistry, the square antiprismatic molecular geometry describes the shape of compounds where eight atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a square antiprism. This shape has D4d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the dodecahedron and the bicapped trigonal prism.
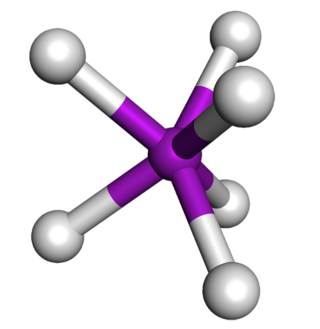
In chemistry, the trigonal prismatic molecular geometry describes the shape of compounds where six atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a triangular prism.

Osmium hexafluoride, also osmium(VI) fluoride, (OsF6) is a compound of osmium and fluorine, and one of the seventeen known binary hexafluorides.

In chemistry, the capped octahedral molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a gyroelongated triangular pyramid. This shape has C3v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped trigonal prism.
Gregory S. Girolami is the William H. and Janet G. Lycan Professor of Chemistry at the University of Illinois at Urbana-Champaign. His research focuses on the synthesis, properties, and reactivity of new inorganic, organometallic, and solid state species. Girolami has been elected a fellow of the American Association for the Advancement of Science, the Royal Society of Chemistry, and the American Chemical Society.
In chemistry, tetradentate ligands are ligands that bind four donor atoms to a central atom to form a coordination complex. This number of donor atoms that bind is called denticity and is a method of classifying ligands.

In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid. This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism.

In chemistry, the bicapped trigonal prismatic molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a biaugmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the dodecahedron.
















