
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those that include transition metals, are coordination complexes.
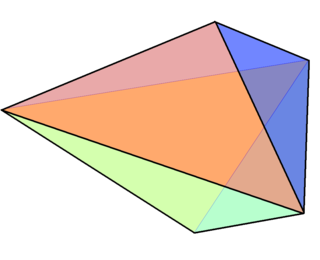
A triangular bipyramid is a hexahedron with six triangular faces constructed by attaching two tetrahedra face-to-face. The same shape is also known as a triangular dipyramid or trigonal bipyramid. If these tetrahedra are regular, all faces of a triangular bipyramid are equilateral. It is an example of a deltahedron, composite polyhedron, and Johnson solid.

Valence shell electron pair repulsion (VSEPR) theory is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.

Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.

In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical, because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride, and phosphorus pentachloride in the gas phase.

In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix octa. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, [Co(NH3)6]3+, which is not octahedral in the mathematical sense due to the orientation of the N−H bonds, is referred to as octahedral.
The coordination geometry of an atom is the geometrical pattern defined by the atoms around the central atom. The term is commonly applied in the field of inorganic chemistry, where diverse structures are observed. The coordination geometry depends on the number, not the type, of ligands bonded to the metal centre as well as their locations. The number of atoms bonded is the coordination number. The geometrical pattern can be described as a polyhedron where the vertices of the polyhedron are the centres of the coordinating atoms in the ligands.

A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant.

Dithiolene metal complexes are complexes containing 1,2-dithiolene ligands. 1,2-Dithiolene ligands, a particular case of 1,2-dichalcogenolene species along with 1,2-diselenolene derivatives, are unsaturated bidentate ligand wherein the two donor atoms are sulfur. 1,2-Dithiolene metal complexes are often referred to as "metal dithiolenes", "metallodithiolenes" or "dithiolene complexes". Most molybdenum- and tungsten-containing proteins have dithiolene-like moieties at their active sites, which feature the so-called molybdopterin cofactor bound to the Mo or W.

Hexamethyltungsten is the chemical compound W(CH3)6 also written WMe6. Classified as a transition metal alkyl complex, hexamethyltungsten is an air-sensitive, red, crystalline solid at room temperature; however, it is extremely volatile and sublimes at −30 °C. Owing to its six methyl groups it is extremely soluble in petroleum, aromatic hydrocarbons, ethers, carbon disulfide, and carbon tetrachloride.

In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group D5h.

The linear molecular geometry describes the geometry around a central atom bonded to two other atoms placed at a bond angle of 180°. Linear organic molecules, such as acetylene, are often described by invoking sp orbital hybridization for their carbon centers.
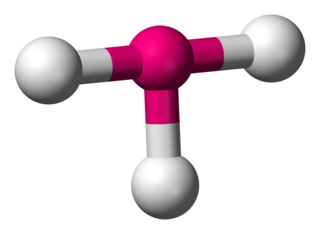
In chemistry, T-shaped molecular geometry describes the structures of some molecules where a central atom has three ligands. Ordinarily, three-coordinated compounds adopt trigonal planar or pyramidal geometries. Examples of T-shaped molecules are the halogen trifluorides, such as ClF3.
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory. However the d electron count of an atom in a complex is often different from the d electron count of a free atom or a free ion of the same element.
In chemistry, molecular oxohalides (oxyhalides) are a group of chemical compounds in which both oxygen and halogen atoms are attached to another chemical element A in a single molecule. They have the general formula AOmXn, where X is a halogen. Known oxohalides have fluorine (F), chlorine (Cl), bromine (Br), and/or iodine (I) in their molecules. The element A may be a main group element, a transition element, a rare earth element or an actinide. The term oxohalide, or oxyhalide, may also refer to minerals and other crystalline substances with the same overall chemical formula, but having an ionic structure.

In chemistry, the tricapped trigonal prismatic molecular geometry describes the shape of compounds where nine atoms, groups of atoms, or ligands are arranged around a central atom, defining the vertices of a triaugmented triangular prism.

In chemistry, the capped octahedral molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a gyroelongated triangular pyramid. This shape has C3v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped trigonal prism.

In chemistry, the capped trigonal prismatic molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of an augmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped octahedron.

In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid. This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism.

In chemistry, the bicapped trigonal prismatic molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a biaugmented triangular prism. This shape has C2v symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the dodecahedron.

2. The intra- and inter-triangle Te-Te distances are 2.70 and 3.06 A, respectively. UJONUE.png](http://upload.wikimedia.org/wikipedia/commons/thumb/8/81/UJONUE.png/220px-UJONUE.png)
















