 | |||
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) | |||
| ChemSpider | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
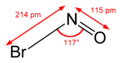
| NOBr | |||
| Molar mass | 109.910 g/mol | ||
| Appearance | Red gas | ||
| Boiling point | 14.5 °C (58.1 °F; 287.6 K) | ||
Refractive index (nD) | 1.524 | ||
| Related compounds | |||
Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Nitrosyl bromide is the chemical compound with the chemical formula NOBr. It is a red gas with a condensation point just below room temperature. [1] It reacts with water. [1]
Contents
- Dissociation kinetics
- Safety Precautions
- Physical characteristics
- References
- Footnotes
- General sources
- External links
Nitrosyl bromide can be formed by the reversible reaction of nitric oxide with bromine. [2] This reaction is of interest as it is one of very few third-order homogeneous gas reactions. NOBr is prone to photodissociation at standard pressure and temperature.
- 2 NO + Br2 ⇌ 2 NOBr
Another way to make it is by way of nitrogen dioxide reacting with potassium bromide. [1]
- 2NO2 + KBr → BrNO + KNO3

