
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted among the rare earth elements; it can also be classified as the first element of the 6th-period transition metals.

Ytterbium is a chemical element; it has symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. Like the other lanthanides, its most common oxidation state is +3, as in its oxide, halides, and other compounds. In aqueous solution, like compounds of other late lanthanides, soluble ytterbium compounds form complexes with nine water molecules. Because of its closed-shell electron configuration, its density, melting point and boiling point are much lower than those of most other lanthanides.

Gold(III) bromide is a dark-red to black crystalline solid. It has the empirical formula AuBr3, but exists as a dimer with the molecular formula Au2Br6 in which two gold atoms are bridged by two bromine atoms. It is commonly referred to as gold(III) bromide, gold tribromide, and rarely but traditionally auric bromide, and sometimes as digold hexabromide. The analogous copper or silver tribromides do not exist.
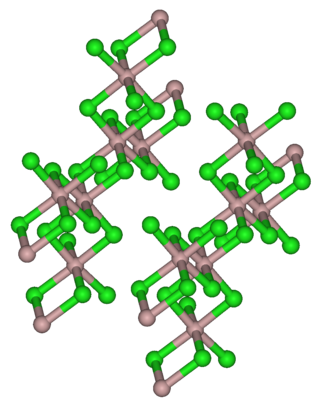
Indium(III) bromide, (indium tribromide), InBr3, is a chemical compound of indium and bromine. It is a Lewis acid and has been used in organic synthesis.
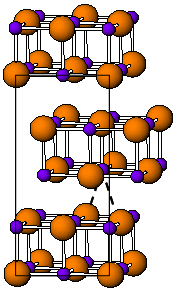
Indium(I) bromide is a chemical compound of indium and bromine. It is a red crystalline compound that is isostructural with β-TlI and has a distorted rock salt structure. Indium(I) bromide is generally made from the elements, heating indium metal with InBr3. It has been used in the sulfur lamp. In organic chemistry, it has been found to promote the coupling of α, α-dichloroketones to 1-aryl-butane-1,4-diones. Oxidative addition reactions with for example alkyl halides to give alkyl indium halides and with NiBr complexes to give Ni-In bonds are known. It is unstable in water decomposing into indium metal and indium tribromide. When indium dibromide is dissolved in water, InBr is produced as a, presumably, insoluble red precipitate, that then rapidly decomposes.

Molybdenum(III) bromide is the inorganic compound with the formula MoBr3. It is a black solid that is insoluble in most solvents but dissolves in donor solvents such as pyridine.
Scandium bromide, or ScBr3, is a trihalide, hygroscopic, water-soluble chemical compound of scandium and bromine.
Praseodymium(III) bromide is a crystalline compound of one praseodymium atom and three bromine atoms.

Neodymium(III) bromide is an inorganic salt of bromine and neodymium the formula NdBr3. The anhydrous compound is an off-white to pale green solid at room temperature, with an orthorhombic PuBr3-type crystal structure. The material is hygroscopic and forms a hexahydrate in water (NdBr3· 6H2O), similar to the related neodymium(III) chloride.
Samarium(III) bromide is a crystalline compound of one samarium and three bromine atoms with the chemical formula of SmBr3. Samarium(III) bromide is a dark brown powder at room temperature. The compound has a crystal structure isotypic to that of plutonium(III) bromide.

Europium(II) bromide is a crystalline compound of one europium atom and two bromine atoms. Europium(II) bromide is a white powder at room temperature, and odorless. Europium dibromide is hygroscopic.
Europium(III) bromide is a crystalline compound, a salt, made of one europium and three bromine atoms. Europium tribromide is a grey powder at room temperature. It is odorless. Europium tribromide is hygroscopic.
Gadolinium(III) bromide is a crystalline compound of gadolinium atoms and three bromine atoms. This salt is hygroscopic.
Holmium(III) bromide is a crystalline compound made of one holmium atom and three bromine atoms. Holmium bromide is a yellow powder at room temperature. Holmium bromide is hygroscopic. Holmium bromide is odorless.

Californium(III) bromide is an inorganic compound, a salt with a chemical formula CfBr3. Like in californium(III) oxide (Cf2O3) and other californium halides, including californium(III) fluoride (CfF3), californium(III) chloride, and californium(III) iodide (CfI3), the californium atom has an oxidation state of +3.

Ruthenium(III) bromide is a chemical compound of ruthenium and bromine with the formula RuBr3. It is a dark brown solid that decomposes above 400 °C.
Praseodymium compounds are compounds formed by the lanthanide metal praseodymium (Pr). In these compounds, praseodymium generally exhibits the +3 oxidation state, such as PrCl3, Pr(NO3)3 and Pr(CH3COO)3. However, compounds with praseodymium in the +2 and +4 oxidation states, and unlike other lanthanides, the +5 oxidation state, are also known.

Zirconium(III) bromide is an inorganic compound with the formula ZrBr3.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state, such as LuCl3, Lu2O3 and Lu2(SO4)3. Aqueous solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts, such as nitrate, sulfate and acetate form hydrates upon crystallization. The oxide, hydroxide, fluoride, carbonate, phosphate and oxalate are insoluble in water.













