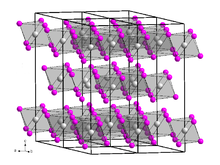
Terbium(III) iodide (TbI3) is an inorganic chemical compound.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.

Antimony triiodide is the chemical compound with the formula SbI3. This ruby-red solid is the only characterized "binary" iodide of antimony, i.e. the sole compound isolated with the formula SbxIy. It contains antimony in its +3 oxidation state. Like many iodides of the heavier main group elements, its structure depends on the phase. Gaseous SbI3 is a molecular, pyramidal species as anticipated by VSEPR theory. In the solid state, however, the Sb center is surrounded by an octahedron of six iodide ligands, three of which are closer and three more distant. For the related compound BiI3, all six Bi—I distances are equal.

Bismuth(III) iodide is the inorganic compound with the formula BiI3. This gray-black salt is the product of the reaction of bismuth and iodine, which once was of interest in qualitative inorganic analysis.
Cerium(III) iodide (CeI3) is the compound formed by cerium(III) cations and iodide anions.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.
Dysprosium(III) nitrate is an inorganic compound, a salt of dysprosium and nitric acid with the chemical formula Dy(NO3)3. The compound forms yellowish crystals, dissolves in water, forms a crystalline hydrate.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3. Neodymium uses the +3 oxidation state in the compound. The anhydrous compound is a green powdery solid at room temperature.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium and iodine, with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.

Hafnium(III) iodide is an inorganic compound of hafnium and iodine with the formula Hf I3. It is a black solid.

Berkelium(III) iodide is a binary inorganic compound of berkelium and iodine with the chemical formula BkI3.

Curium(III) iodide is the chemical compound with the formula CmI3. Since all isotopes of curium are only artificially produced, the compound has no natural occurrence.