Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.
Iodine can form compounds using multiple oxidation states. Iodine is quite reactive, but it is much less reactive than the other halogens. For example, while chlorine gas will halogenate carbon monoxide, nitric oxide, and sulfur dioxide, iodine will not do so. Furthermore, iodination of metals tends to result in lower oxidation states than chlorination or bromination; for example, rhenium metal reacts with chlorine to form rhenium hexachloride, but with bromine it forms only rhenium pentabromide and iodine can achieve only rhenium tetraiodide. By the same token, however, since iodine has the lowest ionisation energy among the halogens and is the most easily oxidised of them, it has a more significant cationic chemistry and its higher oxidation states are rather more stable than those of bromine and chlorine, for example in iodine heptafluoride.
There are three sets of Indium halides, the trihalides, the monohalides, and several intermediate halides. In the monohalides the oxidation state of indium is +1 and their proper names are indium(I) fluoride, indium(I) chloride, indium(I) bromide and indium(I) iodide.

Thallium triiodide is a chemical compound of thallium and iodine with formula TlI3. Unlike the other thallium trihalides, which contain thallium(III), TlI3 is a thallium(I) salt and contains the triiodide ion, I−
3.

In chemistry, a chemical transport reaction describes a process for purification and crystallization of non-volatile solids. The process is also responsible for certain aspects of mineral growth from the effluent of volcanoes. The technique is distinct from chemical vapor deposition, which usually entails decomposition of molecular precursors (e.g. SiH4 → Si + 2 H2) and which gives conformal coatings. The technique, which was popularized by Harald Schäfer, entails the reversible conversion of nonvolatile elements and chemical compounds into volatile derivatives. The volatile derivative migrates throughout a sealed reactor, typically a sealed and evacuated glass tube heated in a tube furnace. Because the tube is under a temperature gradient, the volatile derivative reverts to the parent solid and the transport agent is released at the end opposite to which it originated (see next section). The transport agent is thus catalytic. The technique requires that the two ends of the tube (which contains the sample to be crystallized) be maintained at different temperatures. So-called two-zone tube furnaces are employed for this purpose. The method derives from the Van Arkel de Boer process which was used for the purification of titanium and vanadium and uses iodine as the transport agent.

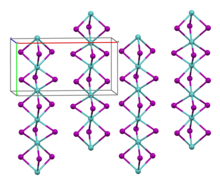
Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.
Tungsten(III) iodide or tungsten triiodide is a chemical compound of tungsten and iodine with the formula WI3.
Iron(III) iodide is an inorganic compound with the chemical formula FeI3. It is a thermodynamically unstable compound that is difficult to prepare. Nevertheless, iron(III) iodide has been synthesised in small quantities in the absence of air and water.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium(III) iodide is an inorganic salt, consisting of the rare-earth metal praseodymium with hydrogen iodide with the chemical formula PrI3. It forms green crystals. It is soluble in water.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+[(I−)2e−].

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
Ruthenium(III) iodide is a chemical compound containing ruthenium and iodine with the formula RuI3. It is a black solid.