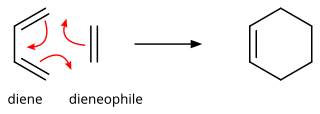
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reaction becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.

Chromium(III) chloride (also called chromic chloride) is an inorganic chemical compound with the chemical formula CrCl3. It forms several hydrates with the formula CrCl3·nH2O, among which are hydrates where n can be 5 (chromium(III) chloride pentahydrate CrCl3·5H2O) or 6 (chromium(III) chloride hexahydrate CrCl3·6H2O). The anhydrous compound with the formula CrCl3 are violet crystals, while the most common form of the chromium(III) chloride are the dark green crystals of hexahydrate, CrCl3·6H2O. Chromium chlorides find use as catalysts and as precursors to dyes for wool.

Scandium(III) chloride is the inorganic compound with the formula ScCl3. It is a white, high-melting ionic compound, which is deliquescent and highly water-soluble. This salt is mainly of interest in the research laboratory. Both the anhydrous form and hexahydrate (ScCl3•6H2O) are commercially available.

Vanadium(III) chloride describes the inorganic compound with the formula VCl3 and its hydrates. It forms a purple anhydrous form and a green hexahydrate [VCl2(H2O)4]Cl·2H2O. These hygroscopic salts are common precursors to other vanadium(III) complexes and is used as a mild reducing agent.
Boron triiodide is a chemical compound of boron and iodine with chemical formula BI3. It has a trigonal planar molecular geometry.

Uranium trifluoride is an inorganic chemical compound with the chemical formula UF3.

Uranium pentachloride is an inorganic chemical compound composed of uranium in the +5 oxidation state and five chlorine atoms.

Uranium(IV) iodide, also known as uranium tetraiodide, is an inorganic chemical compound. It is a salt of uranium in oxidation state +4 and iodine.
Iron(II) iodide is an inorganic compound with the chemical formula FeI2. It is used as a catalyst in organic reactions.
Americium(III) iodide or americium triiodide is the chemical compound, a salt composed of americium and iodine with the formula AmI3.

Indium(III) iodide or indium triiodide is a chemical compound of indium and iodine with the formula InI3.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.
Protactinium(V) bromide is an inorganic compound. It is a halide of protactinium, consisting of protactinium and bromine. It is radioactive and has a chemical formula of PaBr5, which is a red crystal of the monoclinic crystal system.

Protactinium(IV) chloride is an inorganic compound. It is an actinide halide, composed of protactinium and chlorine. It is radioactive, and has the chemical formula of PaCl4. It is a chartreuse-coloured (yellowish-green) crystal of the tetragonal crystal system.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Thulium(III) iodide is an iodide of thulium, with the chemical formula of TmI3. Thulium(III) iodide is used as a component of metal halide lamps.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.
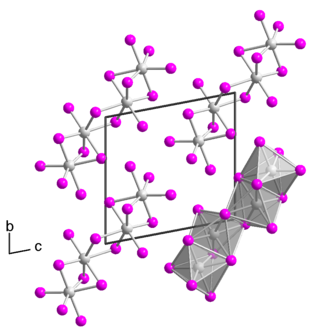
Tantalum(IV) iodide is an inorganic compound with the chemical formula TaI4. It dissolves in water to give a green solution, but the color fades when left in the air and produces a white precipitate.

Tungsten(II) iodide is an iodide of tungsten, with the chemical formula [W6I8]I4, or abbreviated as WI2.
















