Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.
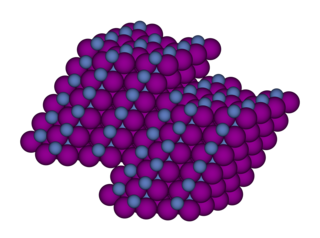
Nickel(II) iodide is an inorganic compound with the formula NiI2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above). This bluish-green colour is typical of hydrated nickel(II) compounds. Nickel iodides find some applications in homogeneous catalysis.

Sodium formate, HCOONa, is the sodium salt of formic acid, HCOOH. It usually appears as a white deliquescent powder.
Terbium(III) iodide (TbI3) is an inorganic chemical compound.

Tellurium tetraiodide (TeI4) is an inorganic chemical compound. It has a tetrameric structure which is different from the tetrameric solid forms of TeCl4 and TeBr4. In TeI4 the Te atoms are octahedrally coordinated and edges of the octahedra are shared.
Magnesium compounds are compounds formed by the element magnesium (Mg). These compounds are important to industry and biology, including magnesium carbonate, magnesium chloride, magnesium citrate, magnesium hydroxide, magnesium oxide, magnesium sulfate, and magnesium sulfate heptahydrate.

Rubidium iodide is a salt of rubidium and iodine, with the chemical formula RbI. It is a white solid with a melting point of 642 °C.
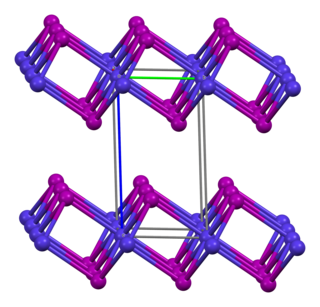
Cobalt(II) iodide or cobaltous iodide are the inorganic compounds with the formula CoI2 and the hexahydrate CoI2(H2O)6. These salts are the principal iodides of cobalt.

Holmium(III) chloride is the inorganic compound with the formula HoCl3. It is a common salt but is mainly used in research. It can be used to produce pure holmium. It exhibits the same color-changing behavior seen in holmium oxide, being a yellow in natural lighting and a bright pink color in fluorescent lighting.
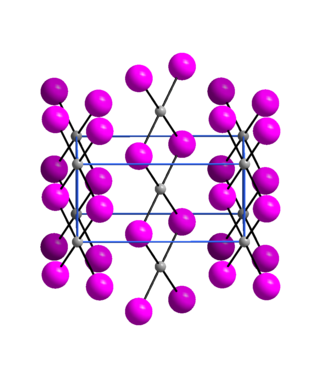
Palladium(II) iodide is an inorganic compound of palladium and iodine. It is commercially available, though less common than palladium(II) chloride, the usual entry point to palladium chemistry. Three polymorphs are known.

Manganese(II) iodide is the chemical compound composed of manganese and iodide with the formula MnI2(H2O)n. The tetrahydrate is a pink solid while the anhydrous derivative is beige. Both forms feature octahedral Mn centers. Unlike MnCl2(H2O)4 and MnBr2(H2O)4 which are cis, MnI2(H2O)4 is trans.

Germanium(II) iodide is an iodide of germanium, with the chemical formula of GeI2.

Germanium(IV) iodide is an inorganic compound with the chemical formula GeI4.

Molybdenum(III) iodide is the inorganic compound with the formula MoI3.
Erbium(III) iodide is an iodide of lanthanide metal erbium. The compound is insoluble in water and is white to slightly pink in appearance.
Gadolinium(III) fluoride is an inorganic compound with a chemical formula GdF3.

Chromium(II) iodide is the inorganic compound with the formula CrI2. It is a red-brown or black solid. The compound is made by thermal decomposition of chromium(III) iodide. Like many metal diiodides, CrI2 adopts the "cadmium iodide structure" motif, i.e., it features sheets of octahedral Cr(II) centers interconnected by bridging iodide ligands. Reflecting the effects of its d4 configuration, chromium's coordination sphere is highly distorted.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.
Europium(III) iodide is an inorganic compound containing europium and iodine with the chemical formula EuI3.

Dysprosium(II) iodide is an iodide of dysprosium with the chemical formula DyI2.













