In chemistry, a half reaction is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. Often, the concept of half reactions is used to describe what occurs in an electrochemical cell, such as a Galvanic cell battery. Half reactions can be written to describe both the metal undergoing oxidation and the metal undergoing reduction.

In chemistry, iron(II) refers to the element iron in its +2 oxidation state. The adjective ferrous or the prefix ferro- is often used to specify such compounds, as in ferrous chloride for iron(II) chloride (FeCl2). The adjective ferric is used instead for iron(III) salts, containing the cation Fe3+. The word ferrous is derived from the Latin word ferrum, meaning "iron".
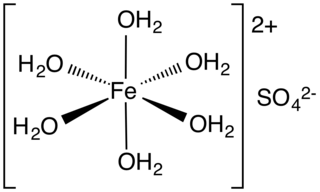
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula Fe SO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but several values for x are known. The hydrated form is used medically to treat or prevent iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.

Zinc sulfate describes a family of inorganic compounds with the formula ZnSO4(H2O)x. All are colorless solids. The most common form includes water of crystallization as the heptahydrate, with the formula ZnSO4·7H2O. As early as the 16th century it was prepared on the large scale, and was historically known as "white vitriol" (the name was used, for example, in 1620s by the collective writing under the pseudonym of Basil Valentine). Zinc sulfate and its hydrates are colourless solids.

Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water.
Iron(II) sulfide or ferrous sulfide is one of a family of chemical compounds and minerals with the approximate formula FeS. Iron sulfides are often iron-deficient non-stoichiometric. All are black, water-insoluble solids.
In horticulture, lime sulfur (lime sulphur in British English, see American and British English spelling differences) is mainly a mixture of calcium polysulfides and thiosulfate (plus other reaction by-products as sulfite and sulfate) formed by reacting calcium hydroxide with elemental sulfur, used in pest control. It can be prepared by boiling in water a suspension of poorly soluble calcium hydroxide (lime) and solid sulfur together with a small amount of surfactant to facilitate the dispersion of these solids in water. After elimination of any residual solids (flocculation, decantation and filtration), it is normally used as an aqueous solution, which is reddish-yellow in colour and has a distinctive offensive odor of hydrogen sulfide (H2S, rotten eggs).

Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)2. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidised solid is sometimes known as "green rust".
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions, as almost all nitrates are soluble in water. In contrast, many common ions give insoluble salts, e.g. halides precipitate with silver, and sulfate precipitate with barium.
In ore deposit geology, supergene processes or enrichment are those that occur relatively near the surface as opposed to deep hypogene processes. Supergene processes include the predominance of meteoric water circulation (i.e. water derived from precipitation) with concomitant oxidation and chemical weathering. The descending meteoric waters oxidize the primary (hypogene) sulfide ore minerals and redistribute the metallic ore elements. Supergene enrichment occurs at the base of the oxidized portion of an ore deposit. Metals that have been leached from the oxidized ore are carried downward by percolating groundwater, and react with hypogene sulfides at the supergene-hypogene boundary. The reaction produces secondary sulfides with metal contents higher than those of the primary ore. This is particularly noted in copper ore deposits where the copper sulfide minerals chalcocite (Cu2S), covellite (CuS), digenite (Cu18S10), and djurleite (Cu31S16) are deposited by the descending surface waters.

Ettringite is a hydrous calcium aluminium sulfate mineral with formula: Ca6Al2(SO4)3(OH)12·26H2O. It is a colorless to yellow mineral crystallizing in the trigonal system. The prismatic crystals are typically colorless, turning white on partial dehydration. It is part of the ettringite-group which includes other sulfates such as thaumasite and bentorite.
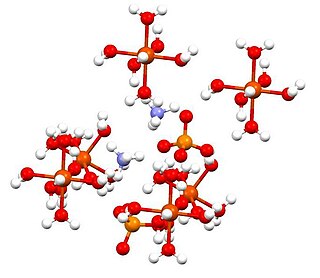
Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2Fe(SO4)2(H2O)6. Containing two different cations, Fe2+ and NH+4, it is classified as a double salt of ferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry. Its mineral form is mohrite.

Iron(III) sulfate (or ferric sulfate), is a family of inorganic compounds with the formula Fe2(SO4)3(H2O)n. A variety of hydrates are known, including the most commonly encountered form of "ferric sulfate". Solutions are used in dyeing as a mordant, and as a coagulant for industrial wastes. Solutions of ferric sulfate are also used in the processing of aluminum and steel.

Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known, the hemihydrate and the tetrahydrate, respectively CaSO3·½(H2O) and CaSO3·4(H2O). All forms are white solids. It is most notable as the product of flue-gas desulfurization.

Ammonium iron(III) sulfate, NH4Fe(SO4)2·12 H2O, or NH4[Fe(H2O)6](SO4)2·6 H2O, also known as ferric ammonium sulfate (FAS) or iron alum, is a double salt in the class of alums, which consists of compounds with the general formula AB(SO4)2 · 12 H2O. It has the appearance of weakly violet, octahedrical crystals. There has been some discussion regarding the origin of the crystals' colour, with some ascribing it to impurities in the compound, and others claiming it to be a property of the crystal itself.

Concrete degradation may have many different causes. Concrete is mostly damaged by the corrosion of reinforcement bars due to the carbonatation of hardened cement paste or chloride attack under wet conditions. Chemical damages are caused by the formation of expansive products produced by various chemical reactions, by aggressive chemical species present in groundwater and seawater, or by microorganisms. Other damaging processes can also involve calcium leaching by water infiltration and different physical phenomena initiating cracks formation and propagation. All these detrimental processes and damaging agents adversely affects the concrete mechanical strength and its durability.
Iron(II) selenate (ferrous selenate) is an inorganic compound with the formula FeSeO4. It has anhydrous and several hydrate forms. The pentahydrate has the structure, [Fe(H2O)4]SeO4•H2O, isomorphous to the corresponding iron(II) sulfate. Heptahydrate is also known, in form of unstable green crystalline solid.

Ferric EDTA is the coordination complex formed from ferric ions and EDTA. EDTA has a high affinity for ferric ions. It gives yellowish aqueous solutions.

Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however it is not commercially available unlike iron(III) nitrate due to its instability to air. The salt is soluble in water serves as a ready source of ferrous ions.














