
An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
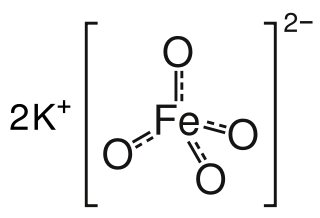
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.

Copper chromite often refers to inorganic compounds with the formula Cu2Cr2Ox. They are black solids. Cu2Cr2O4 is a well-defined material. The other copper chromite often is described as Cu2Cr2O5. It is used to catalyze reactions in organic chemistry.
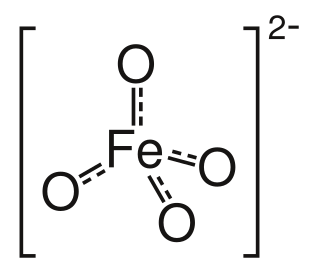
Ferrate(VI) is the inorganic anion with the chemical formula [FeO4]2−. It is photosensitive, contributes a pale violet colour to compounds and solutions containing it and is one of the strongest water-stable oxidizing species known. Although it is classified as a weak base, concentrated solutions containing ferrate(VI) are corrosive and attack the skin and are only stable at high pH. It is similar to the somewhat more stable permanganate.

Potassium chromate is the inorganic compound with the formula K2CrO4. This yellow solid is the potassium salt of the chromate anion. It is a common laboratory chemical, whereas sodium chromate is important industrially.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.
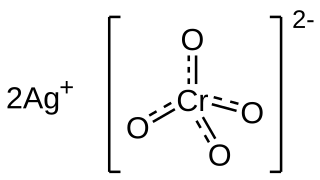
Silver chromate is an inorganic compound with formula Ag2CrO4 which appears as distinctively coloured brown-red crystals. The compound is insoluble and its precipitation is indicative of the reaction between soluble chromate and silver precursor salts (commonly potassium/sodium chromate with silver nitrate). This reaction is important for two uses in the laboratory: in analytical chemistry it constitutes the basis for the Mohr method of argentometry, whereas in neuroscience it is used in the Golgi method of staining neurons for microscopy.

Caesium chromate or cesium chromate is an inorganic compound with the formula Cs2CrO4. It is a yellow crystalline solid that is the caesium salt of chromic acid, and it crystallises in the orthorhombic system.

Iron(III) nitrate, or ferric nitrate, is the name used for a series of inorganic compounds with the formula Fe(NO3)3.(H2O)n. Most common is the nonahydrate Fe(NO3)3.(H2O)9. The hydrates are all pale colored, water-soluble paramagnetic salts.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

Potassium chlorochromate is an inorganic compound with the formula KCrO3Cl. It is the potassium salt of chlorochromate, [CrO3Cl]−. It is a water-soluble orange compound is used occasionally for oxidation of organic compounds. It is sometimes called Péligot's salt, in recognition of its discoverer Eugène-Melchior Péligot.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores.
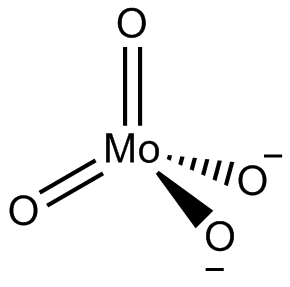
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of +6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
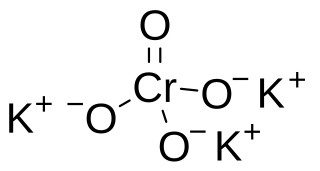
Potassium hypochromate is a chemical compound with the formula K3CrO4 with the unusual Cr5+ ion. This compound is unstable in water but stable in alkaline solution and was found to have a similar crystal structure to potassium hypomanganate.
Europium(III) chromate is a chemical compound composed of europium, chromium and oxygen with europium in the +3 oxidation state, chromium in the +5 oxidation state and oxygen in the −2 oxidation state. It has the chemical formula of EuCrO4.














