Related Research Articles

Chromium is a chemical element; it has symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
Chromic acid is jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
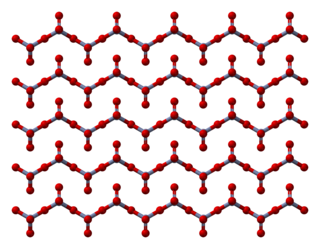
Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions and bright orange when wet. The substance dissolves in water accompanied by hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser, a mutagen, and a carcinogen.
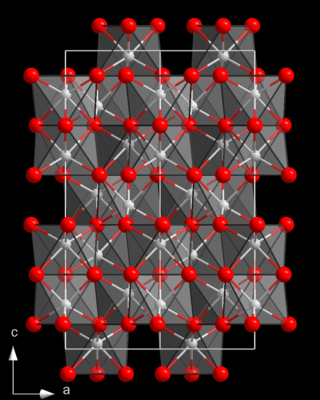
Chromium(III) oxide is an inorganic compound with the formula Cr
2O
3. It is one of the principal oxides of chromium and is used as a pigment. In nature, it occurs as the rare mineral eskolaite.

Copper chromite often refers to inorganic compounds with the formula Cu2Cr2Ox. They are black solids. Cu2Cr2O4 is a well-defined material. The other copper chromite often is described as Cu2Cr2O5. It is used to catalyze reactions in organic chemistry.

Sodium dichromate is the inorganic compound with the formula Na2Cr2O7. However, the salt is usually handled as its dihydrate Na2Cr2O7·2H2O. Virtually all chromium ore is processed via conversion to sodium dichromate and virtually all compounds and materials based on chromium are prepared from this salt. In terms of reactivity and appearance, sodium dichromate and potassium dichromate are very similar. The sodium salt is, however, around twenty times more soluble in water than the potassium salt (49 g/L at 0 °C) and its equivalent weight is also lower, which is often desirable.

Caesium chromate or cesium chromate is an inorganic compound with the formula Cs2CrO4. It is a yellow crystalline solid that is the caesium salt of chromic acid, and it crystallises in the orthorhombic system.

Chromium compounds are compounds containing the element chromium (Cr). Chromium is a member of group 6 of the transition metals. The +3 and +6 states occur most commonly within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist.

Nickel(II) chromate (NiCrO4) is an acid-soluble compound, red-brown in color, with high tolerances for heat. It and the ions that compose it have been linked to tumor formation and gene mutation, particularly to wildlife.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores.
Iron(III) chromate is the iron(III) salt of chromic acid with the chemical formula Fe2(CrO4)3.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones. The reaction was an early method for the oxidation of alcohols. Its use has subsided because milder, more selective reagents have been developed, e.g. Collins reagent.
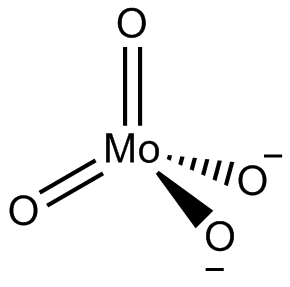
In chemistry, a molybdate is a compound containing an oxyanion with molybdenum in its highest oxidation state of 6: O−−Mo(=O)2−O−. Molybdenum can form a very large range of such oxyanions, which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state. The larger oxyanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxyanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
A chromate ester is a chemical structure that contains a chromium atom (symbol Cr) in a +6 oxidation state that is connected via an oxygen (O) linkage to a carbon (C) atom. The Cr itself is in its chromate form, with several oxygens attached, and the Cr–O–C attachment makes this chemical group structurally similar to other ester functional groups. They can be synthesized from various chromium(VI) metal compounds, such as CrO3, chromium chloride complexes, and aqueous chromate ions, and tend to react via redox reactions to liberate chromium(IV).

The chromium cycle is the biogeochemical cycle of chromium through the atmosphere, hydrosphere, biosphere and lithosphere.
Potassium hypochromate is a chemical compound with the formula K3CrO4 with the unusual Cr5+ ion. This compound is unstable in water but stable in alkaline solution and was found to have a similar crystal structure to potassium hypomanganate.
References
- ↑ G. Buisson, E. F. Bertaut, J. Mareschal (1964), "Etude cristallographique des composes TCrO4 (T = terre rare ou Y)", Comptes rendus hebdomadaires des séances de l'Académie des Sciences (in German), vol. 259, pp. 411–413
{{citation}}: CS1 maint: multiple names: authors list (link) - 1 2 3 Hidetaka Konno, Yoshitaka Aoki, Zoltán Klencsár, Attila Vértes, Makoto Wakeshima (2001-12-01). "Structure of EuCrO4 and Its Electronic and Magnetic Properties". Bulletin of the Chemical Society of Japan. 74 (12): 2335–2341. doi:10.1246/bcsj.74.2335. ISSN 0009-2673.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 3 J. Thakur, R. Shukla, N. Raje, D. Ghonge, H. Bagla (2011-10-01). "Synthesis, Structural Characterization and Thermal Stability of Nanocrystalline Rare-Earth Chromates (RECrO4) and Rare-Earth Chromites (RECrO3)". Nanoscience and Nanotechnology Letters. 3 (5): 648–654. doi:10.1166/nnl.2011.1233.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - 1 2 3 E Jiménez, J Isasi, R Sáez-Puche (2000-11-01). "Synthesis, structural characterization and magnetic properties of RCrO4 oxides, R=Nd, Sm, Eu and Lu". Journal of Alloys and Compounds. 312 (1–2): 53–59. doi:10.1016/S0925-8388(00)01079-3.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Yoshitaka Aoki, Hidetaka Konno, Hiroto Tachikawa, Michio Inagaki (2000-05-01). "Characterization of LaCrO4 and NdCrO4 by XRD, Raman Spectroscopy, and ab Initio Molecular Orbital Calculations". Bulletin of the Chemical Society of Japan. 73 (5): 1197–1203. doi:10.1246/bcsj.73.1197. ISSN 0009-2673.
{{cite journal}}: CS1 maint: multiple names: authors list (link)