
Yttrium arsenide is an inorganic compound of yttrium and arsenic with the chemical formula YAs. It can be prepared by reacting yttrium and arsenic at high temperature. Some literature has done research on the eutectic system of it and zinc arsenide.

Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.
Calcium selenide (CaSe) is a chemical compound consisting of the elements calcium and selenium in equal stoichiometric ratio.
Yttrium(III) sulfate is an inorganic compound with the formula Y2(SO4)3. The most common form is the anhydrate and octahydrate.

Europium(II) chloride is an inorganic compound with a chemical formula EuCl2. When it is irradiated by ultraviolet light, it has bright blue fluorescence.

Lutetium(III) hydroxide is an inorganic compound with the chemical formula Lu(OH)3.
Scandium(III) hydroxide is an inorganic compound with the chemical formula Sc(OH)3, the trivalent hydroxide of scandium. It is an amphoteric compound. It is slightly soluble in water, and its saturated solution contains Sc(OH)3 and a small amount of Sc(OH)+2. The solubility of scandium(III) hydroxide in water is 0.0279 mol/L. It will convert to ScO(OH) after aging, greatly reducing the solubility (0.0008 mol/L). Scandium(III) hydroxide can be produced by reacting scandium salts and alkali hydroxides. In the reaction, different starting ingredients can generate different intermediates such as Sc(OH)1.75Cl1.25, Sc(OH)2NO3 and Sc(OH)2.32(SO4)0.34.

Neodymium perrhenate is an inorganic compound with the chemical formula Nd(ReO4)3, which exists in anhydrous and tetrahydrate. It can be obtained by reacting excess neodymium oxide with 240 g/L perrhenic acid solution. In its solution, NdReO42+ and Nd(ReO4)2+ can be observed with stability constants of 16.5 and 23.6, respectively.
Neodymium molybdate is an inorganic compound, with the chemical formula of Nd2(MoO4)3.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Many europium compounds fluoresce under ultraviolet light due to the excitation of electrons to higher energy levels. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

Neodymium(III) phosphate is an inorganic compound, with the chemical formula of NdPO4. Its hemihydrate can be obtained by the reaction of neodymium(III) chloride and phosphoric acid; its anhydrous form can be obtained by the reaction of silicon pyrophosphate (SiP2O7) and neodymium(III) fluoride. It reacts with calcium pyrophosphate to obtain Ca9Nd(PO4)7.
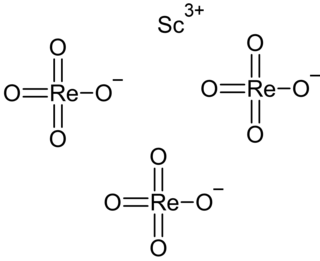
Scandium perrhenate is an inorganic compound, with the chemical formula Sc(ReO4)3. Its thermal stability is lower than that of the corresponding compounds of the yttrium and lanthanum perrhenates.
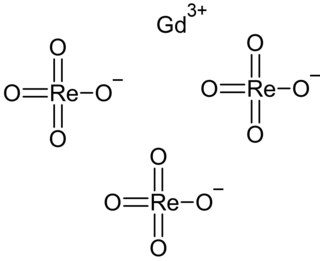
Gadolinium perrhenate is an inorganic compound, with the chemical formula of Gd(ReO4)3. It can be obtained by dissolving an excess of gadolinium oxide in a perrhenic acid solution (240 g/L) in the presence of hydrogen peroxide, from which the hydrates are precipitated. Its tetrahydrate loses water by heating to obtain the anhydrous form, which then decomposes at high temperatures to generate gadolinium oxide and rhenium heptoxide.

Lithium tellurite is an inorganic compound, with the chemical formula Li2TeO3. It crystallises in the monoclinic crystal system, with space group C2/c. It can be prepared by reacting lithium oxide, lithium hydroxide or lithium carbonate with tellurium dioxide. It reacts with lithium fluoride at high temperatures in a 3:1 stoichiometric ratio to obtain Li7(TeO3)3F.

Samarium(III) phosphate is an inorganic compound, with the chemical formula of SmPO4. It is one of the phosphates of samarium.
Lutetium selenide is an inorganic compound with the chemical formula Lu2Se3. It can be obtained by reacting lutetium and selenium or lutetium oxide and hydrogen selenide at a high temperature. It can form orthorhombic AgLuSe2 in the binary system of silver selenide. It can form Lu2PbSe4 and Lu2Pb4Se7 in the binary system of lead selenide.
Thulium selenide is an inorganic compound with the chemical formula Tm2Se3. It can be obtained by the reaction of thulium and selenium or thulium oxide and hydrogen selenide at high temperature, or generated in the thermal decomposition of (py)3Tm(SePh)3. In the binary system of it and antimony triselenide, TmSb3Se6, Tm6Sb8Se21, TmSbSe3 and Tm8Sb2Se15 can be formed.
Dysprosium selenide is one of the selenides of dysprosium, with the chemical formula of Dy2Se3. It can be prepared by reacting dysprosium(III) oxide or dysprosium(III) chloride with hydrogen selenide, or by reacting the elements at high temperatures. It reacts with arsenic triselenide to obtain DyAsSe3.
Germanium diselenide is an inorganic compound, with the chemical formula of GeSe2.

Thulium(III) selenate is an inorganic compound, with the chemical formula Tm2(SeO4)3. It can be obtained by reacting a thulium(III) oxide and selenic acid solution and crystallizing it. It crystallises with ammonium selenate in an aqueous solution to obtain NH4Tm(SeO4)2·3H2O.










