Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.
A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes (alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, cups, jars, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.
Robert L. Banks was an American chemist. He was born and grew up in Piedmont, Missouri. He attended the University of Missouri, Rolla, and initiated into Alpha Phi Omega in 1940. He joined the Phillips Petroleum company in 1946 and worked there until he retired in 1985. He died in Missouri on January 3, 1989.

In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.
A post-metallocene catalyst is a kind of catalyst for the polymerization of olefins, i.e., the industrial production of some of the most common plastics. "Post-metallocene" refers to a class of homogeneous catalysts that are not metallocenes. This area has attracted much attention because the market for polyethylene, polypropylene, and related copolymers is large. There is a corresponding intense market for new processes as indicated by the fact that, in the US alone, 50,000 patents were issued between 1991-2007 on polyethylene and polypropylene.

Heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reagents or products. The process contrasts with homogeneous catalysis where the reagents, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.

In organic chemistry, olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry.
Coking is the process of heating coal in the absence of oxygen to a temperature above 600 °C (1,112 °F) to drive off the volatile components of the raw coal, leaving behind a hard, strong, porous material with a high carbon content called coke. Coke is predominantly carbon. Its porous structure provides a high surface area, allowing it to burn more rapidly, much like how a bundle of tinder burns faster than a solid wooden log. As such, when a kilogram of coke is burned, it releases more heat than a kilogram of the original coal.
John Paul Hogan was an American research chemist. Along with Robert Banks, he discovered methods of producing polypropylene and high-density polyethylene.

Silicotungstic acid or tungstosilicic acid is a heteropoly acid with the chemical formula H4[SiW12O40]. It forms hydrates H4[SiW12O40]·nH2O. In freshly prepared samples, n is approximately 29, but after prolonged desiccation, n = 6. It is a white solid although impure samples appear yellow. It is used as a catalyst in the chemical industry.
Catalyst poisoning is the partial or total deactivation of a catalyst by a chemical compound. Poisoning refers specifically to chemical deactivation, rather than other mechanisms of catalyst degradation such as thermal decomposition or physical damage. Although usually undesirable, poisoning may be helpful when it results in improved catalyst selectivity. An important historic example was the poisoning of catalytic converters by leaded fuel.

Jean-Marie Basset is a French chemist, and is currently the director of KAUST catalysis research center.
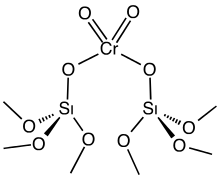
Organochromium chemistry is a branch of organometallic chemistry that deals with organic compounds containing a chromium to carbon bond and their reactions. The field is of some relevance to organic synthesis. The relevant oxidation states for organochromium complexes encompass the entire range of possible oxidation states from –4 (d10) in Na4[Cr–IV(CO)4] to +6 (d0) in oxo-alkyl complexes like Cp*CrVI(=O)2Me.

James Carl Stevens, a chemist, was the first Distinguished Fellow, at the Dow Chemical Company, retiring in January 2015. His area of expertise is organometallic chemistry and his primary field of research is in the area of polyolefin catalysis, particularly in the area of polyethylene, polypropylene, ethylene/styrene copolymers, and the combinatorial discovery of organometallic single-site catalysts. Stevens major contributions have come in the discovery and commercial implementation of single-site polyolefin catalysts. He invented and led the commercialization of constrained geometry catalyst for the polymerization of olefins. These have been commercialized by Dow as a number of polymers, elastomers and plostomers.
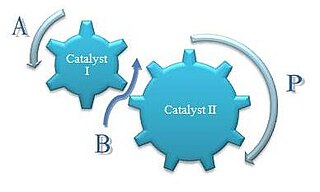
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.

In chemistry, a catalyst support is a material, usually a solid with a high surface area, to which a catalyst is affixed. The activity of heterogeneous catalysts is mainly promoted by atoms present at the accessible surface of the material. Consequently, great effort is made to maximize the specific surface area of a catalyst. One popular method for increasing surface area involves distributing the catalyst over the surface of the support. The support may be inert or participate in the catalytic reactions. Typical supports include various kinds of activated carbon, alumina, and silica.
In polymer chemistry, chain walking (CW) or chain running or chain migration is a mechanism that operates during some alkene polymerization reactions. CW can be also considered as a specific case of intermolecular chain transfer. This reaction gives rise to branched and hyperbranched/dendritic hydrocarbon polymers. This process is also characterized by accurate control of polymer architecture and topology. The extent of CW, displayed in the number of branches formed and positions of branches on the polymers are controlled by the choice of a catalyst. The potential applications of polymers formed by this reaction are diverse, from drug delivery to phase transfer agents, nanomaterials, and catalysis.
Diiminopyridines are a class of diimine ligands. They featuring a pyridine nucleus with imine sidearms appended to the 2,6–positions. The three nitrogen centres bind metals in a tridentate fashion, forming pincer complexes. Diiminopyridines are notable as non-innocent ligand that can assume more than one oxidation state. Complexes of DIPs participate in a range of chemical reactions, including ethylene polymerization, hydrosilylation, and hydrogenation.