
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it is found in various minerals, such as xenotime. Naturally occurring dysprosium is composed of seven isotopes, the most abundant of which is 164Dy.

Samarium(II) iodide is an inorganic compound with the formula SmI2. When employed as a solution for organic synthesis, it is known as Kagan's reagent. SmI2 is a green solid and solutions are green as well. It is a strong one-electron reducing agent that is used in organic synthesis.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction".
Nickel(II) iodide is an inorganic compound with the formula NiI2. This paramagnetic black solid dissolves readily in water to give bluish-green solutions, from which crystallizes the aquo complex [Ni(H2O)6]I2 (image above). This bluish-green colour is typical of hydrated nickel(II) compounds. Nickel iodides find some applications in homogeneous catalysis.

Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the extremely rare mineral coccinite. Unlike the related mercury(II) chloride it is hardly soluble in water (<100 ppm).
Cobalt(II) iodide or cobaltous iodide are the inorganic compounds with the formula CoI2 and the hexahydrate CoI2(H2O)6. These salts are the principal iodides of cobalt.
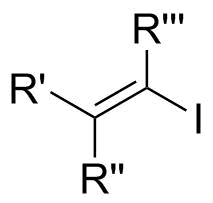
In organic chemistry, a vinyl iodide functional group is an alkene with one or more iodide substituents. Vinyl iodides are versatile molecules that serve as important building blocks and precursors in organic synthesis. They are commonly used in carbon-carbon forming reactions in transition-metal catalyzed cross-coupling reactions, such as Stille reaction, Heck reaction, Sonogashira coupling, and Suzuki coupling. Synthesis of well-defined geometry or complexity vinyl iodide is important in stereoselective synthesis of natural products and drugs.

Europium(II) iodide is the iodide salt of divalent europium cation.

Neodymium(II) iodide or neodymium diiodide is an inorganic salt of iodine and neodymium the formula NdI2. Neodymium uses the +2 oxidation state in the compound.

Praseodymium diiodide is a chemical compound with the empirical formula of PrI2, consisting of praseodymium and iodine. It is an electride, with the ionic formula of Pr3+(I−)2e−, and therefore not a true praseodymium(II) compound.

Lanthanum(III) iodide is an inorganic compound containing lanthanum and iodine with the chemical formula LaI
3.

Dysprosium(III) bromide is an inorganic compound of bromine and dysprosium, with the chemical formula of DyBr3.

Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3.

Gadolinium(III) iodide is an iodide of gadolinium, with the chemical formula of GdI3. It is a yellow, highly hygroscopic solid with a bismuth(III) iodide-type crystal structure. In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.

Thulium(III) iodide is an iodide of thulium, with the chemical formula of TmI3. Thulium(III) iodide is used as a component of metal halide lamps.

Lanthanum diiodide is an iodide of lanthanum, with the chemical formula of LaI2. It is an electride, actually having a chemical formula of La3+[(I−)2e−].

Cerium diiodide is an iodide of cerium, with the chemical formula of CeI2.

Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.

Dysprosium(III) iodide is a binary inorganic compound of dysprosium and iodine with the chemical formula DyI
3.











