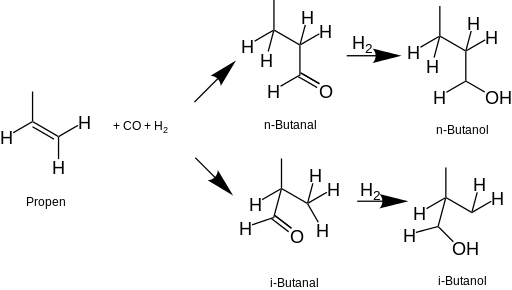
Isomers
The unmodified term butanol usually refers to the straight chain isomer with the alcohol functional group at the terminal carbon, which is also known as 1-butanol. The straight chain isomer with the alcohol at an internal carbon is sec-butyl alcohol or 2-butanol. The branched isomer with the alcohol at a terminal carbon is isobutanol or 2-methyl-1-propanol, and the branched isomer with the alcohol at the internal carbon is tert-butyl alcohol or 2-methyl-2-propanol.
| |  | 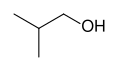 |  |
| 1-Butanol (n-butanol) | 2-Butanol (sec-butyl alcohol) | Isobutanol (2-methylpropan-1-ol) | tert-butyl alcohol (2,2-methylethanol) |
The butanol isomers have different melting and boiling points. 1-Butanol and isobutanol have limited solubility, sec-butyl alcohol has substantially greater solubility, whereas tert-butyl alcohol is miscible with water. The hydroxyl group makes the molecule polar, promoting solubility in water, while the longer hydrocarbon chain mitigates the polarity and reduces solubility.