
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc).

Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose, fructose, and galactose. Compound sugars, also called disaccharides or double sugars, are molecules made of two bonded monosaccharides; common examples are sucrose, lactose, and maltose. White sugar is a refined form of sucrose. In the body, compound sugars are hydrolysed into simple sugars.

Sucralose is an artificial sweetener and sugar substitute. As the majority of ingested sucralose is not metabolized by the body, it adds very little food energy. In the European Union, it is also known under the E number E955. It is produced by chlorination of sucrose, selectively replacing three of the hydroxy groups—in the C1 and C6 positions of the fructose portion and the C4 position of the glucose portion—to give a 1,6-dichloro-1,6-dideoxyfructose–4-chloro-4-deoxygalactose disaccharide. Sucralose is about 600 times sweeter than sucrose, 3 times as sweet as both aspartame and acesulfame potassium, and 2 times as sweet as sodium saccharin.

Fructose, or fruit sugar, is a ketonic simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galactose, that are absorbed by the gut directly into the blood of the portal vein during digestion. The liver then converts both fructose and galactose into glucose, so that dissolved glucose, known as blood sugar, is the only monosaccharide present in circulating blood.
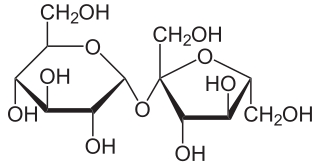
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula C
12H
22O
11.

Sorbitol, less commonly known as glucitol, is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol group (−CH2OH). Most sorbitol is made from potato starch, but it is also found in nature, for example in apples, pears, peaches, and prunes. It is converted to fructose by sorbitol-6-phosphate 2-dehydrogenase. Sorbitol is an isomer of mannitol, another sugar alcohol; the two differ only in the orientation of the hydroxyl group on carbon 2. While similar, the two sugar alcohols have very different sources in nature, melting points, and uses.

A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets.

Xylitol is a chemical compound with the formula C
5H
12O
5, or HO(CH2)(CHOH)3(CH2)OH; specifically, one particular stereoisomer with that structural formula. It is a colorless or white crystalline solid that is freely soluble in water. It is classified as a polyalcohol and a sugar alcohol, specifically an alditol. The name derives from Ancient Greek: ξύλον, xyl[on] 'wood', with the suffix -itol used to denote it being a sugar alcohol.

In cooking, syrup is a condiment that is a thick, viscous liquid consisting primarily of a solution of sugar in water, containing a large amount of dissolved sugars but showing little tendency to deposit crystals. In its concentrated form, its consistency is similar to that of molasses. The viscosity arises from the multiple hydrogen bonds between the dissolved sugar, which has many hydroxyl (OH) groups.
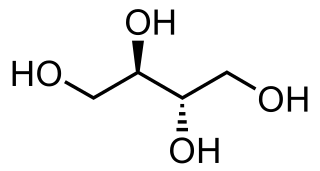
Sugar alcohols are organic compounds, typically derived from sugars, containing one hydroxyl group (−OH) attached to each carbon atom. They are white, water-soluble solids that can occur naturally or be produced industrially by hydrogenating sugars. Since they contain multiple (−OH) groups, they are classified as polyols.

Erythritol (, ) is an organic compound, the naturally occurring achiral meso four-carbon sugar alcohol (or polyol). It is the reduced form of either D- or L-erythrose and one of the two reduced forms of erythrulose. It is used as a food additive and sugar substitute. It is synthesized from corn using enzymes and fermentation. Its formula is C
4H
10O
4, or HO(CH2)(CHOH)2(CH2)OH.

Mannitol is a type of sugar alcohol used as a sweetener and medication. It is used as a low calorie sweetener as it is poorly absorbed by the intestines. As a medication, it is used to decrease pressure in the eyes, as in glaucoma, and to lower increased intracranial pressure. Medically, it is given by injection or inhalation. Effects typically begin within 15 minutes and last up to 8 hours.

Lactitol is a disaccharide sugar alcohol produced from lactose. It is used as a replacement bulk sweetener for low calorie foods with 30–40% of the sweetness of sucrose. It is also used medically as a laxative.

Tagatose is a hexose monosaccharide. It is found in small quantities in a variety of foods, and has attracted attention as an alternative sweetener. It is often found in dairy products, because it is formed when milk is heated. It is similar in texture and appearance to sucrose :215 and is 92% as sweet,:198 but with only 38% of the calories.:209 Tagatose is generally recognized as safe by the Food and Agriculture Organization and the World Health Organization, and has been since 2001. Since it is metabolized differently from sucrose, tagatose has a minimal effect on blood glucose and insulin levels. Tagatose is also approved as a tooth-friendly ingredient for dental products. Consumption of more than about 30 grams of tagatose in a dose may cause gastric disturbance in some people, as it is mostly processed in the large intestine, similar to soluble fiber.:214

High-fructose corn syrup (HFCS), also known as glucose–fructose, isoglucose and glucose–fructose syrup, is a sweetener made from corn starch. As in the production of conventional corn syrup, the starch is broken down into glucose by enzymes. To make HFCS, the corn syrup is further processed by D-xylose isomerase to convert some of its glucose into fructose. HFCS was first marketed in the early 1970s by the Clinton Corn Processing Company, together with the Japanese Agency of Industrial Science and Technology, where the enzyme was discovered in 1965.

Isomalt is a sugar substitute, a mixture of the two disaccharide alcohols 1,6-GPS and 1,1-GPM. It is used primarily for its sugar-like physical properties. It has little to no impact on blood sugar levels, and does not stimulate the release of insulin. It also does not promote tooth decay and is considered to be tooth-friendly. Its energy value is 2 kcal per gram, half that of sugars. It is less sweet than sugar, but can be blended with high-intensity sweeteners such as sucralose to create a mixture with the same sweetness as sucrose (‘sugar’).

D-Psicose (C6H12O6), also known as D-allulose, or simply allulose, is a low-calorie epimer of the monosaccharide sugar fructose, used by some major commercial food and beverage manufacturers as a low-calorie sweetener. First identified in wheat in the 1940s, allulose is naturally present in small quantities in certain foods.

Isomaltulose is a disaccharide carbohydrate composed of glucose and fructose. It is naturally present in honey and sugarcane extracts and is also produced industrially from table sugar (sucrose) and used as a sugar alternative.
Lycasin is a trade name given by Roquette for hydrogenated glucose syrup. One of the major components of Lycasin is maltitol, derived from the hydrogenation of maltose. Depending on the dextrose equivalent (DE) of the syrup used in the hydrolysis, a variety of products can be made, with the name "lycasin" normally being reserved for lycasin 80/55. The other grades are referred to as Polysorb, but should not be confused with the polyglycolic acid suture of the same name which is produced by a different company.
Hydrogenated starch hydrolysates (HSHs), also known as polyglycitol syrup, are mixtures of several sugar alcohols. Hydrogenated starch hydrolysates were developed by the Swedish company Lyckeby Starch in the 1960s. The HSH family of polyols is an approved food ingredient in Canada, Japan, and Australia. HSH sweeteners provide 40 to 90% sweetness relative to table sugar.

















