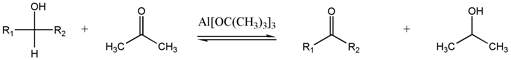Alcohol oxidation is a collection of oxidation reactions in organic chemistry that convert alcohols to aldehydes, ketones, carboxylic acids, and esters. The reaction mainly applies to primary and secondary alcohols. Secondary alcohols form ketones, while primary alcohols form aldehydes or carboxylic acids. [1]
Contents
- To aldehydes and ketones
- In industry
- Laboratory
- Oxidation of diols
- To carboxylic acids
- In industry 2
- Potassium permanganate
- Jones oxidation
- Two-step oxidation of alcohols to acids via isolated aldehydes
- Niche methods and reagents
- References
A variety of oxidants can be used.
Almost all industrial scale oxidations use oxygen or air as the oxidant. [2]
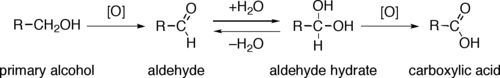
Through a variety of mechanisms, the removal of a hydride equivalent converts a primary or secondary alcohol to an aldehyde or ketone, respectively. The oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (gem-diol, R-CH(OH)2) by reaction with water. Thus, the oxidation of a primary alcohol at the aldehyde level without further oxidation to the carboxylic acid is possible by performing the reaction in absence of water, so that no aldehyde hydrate can be formed.

Oxidation of alcohols to aldehydes and ketones