| Skraup reaction | |
|---|---|
| Named after | Zdenko Hans Skraup |
| Reaction type | Ring forming reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000062 |
The Skraup synthesis is a chemical reaction used to synthesize quinolines. It is named after the Czech chemist Zdenko Hans Skraup (1850-1910). In the archetypal Skraup reaction, aniline is heated with sulfuric acid, glycerol, and an oxidizing agent such as nitrobenzene to yield quinoline. [1] [2] [3] [4]

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.

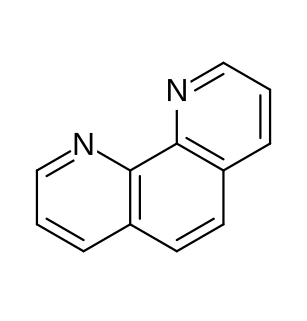
Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

Zdenko Hans Skraup was a Czech-Austrian chemist who discovered the Skraup reaction, the first quinoline synthesis.

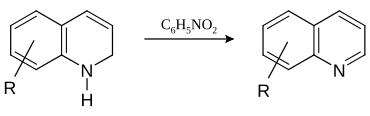
In this example, nitrobenzene serves as both the solvent and the oxidizing agent. The reaction, which otherwise has a reputation for being violent, is typically conducted in the presence of ferrous sulfate. [5] Arsenic acid may be used instead of nitrobenzene and the former is better since the reaction is less violent. [6]

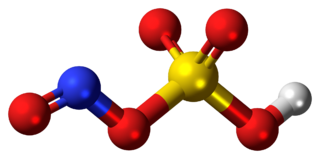
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

A solvent is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Common uses for organic solvents are in dry cleaning, as paint thinners, as nail polish removers and glue solvents, in spot removers, in detergents and in perfumes (ethanol). Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within a cell. Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes.
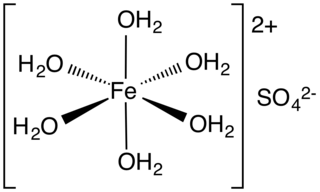
Iron(II) sulfate (British English: iron(II) sulphate) or ferrous sulfate denotes a range of salts with the formula FeSO4·xH2O. These compounds exist most commonly as the heptahydrate (x = 7) but are known for several values of x. The hydrated form is used medically to treat iron deficiency, and also for industrial applications. Known since ancient times as copperas and as green vitriol (vitriol is an archaic name for sulfate), the blue-green heptahydrate (hydrate with 7 molecules of water) is the most common form of this material. All the iron(II) sulfates dissolve in water to give the same aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry and is paramagnetic. The name copperas dates from times when the copper(II) sulfate was known as blue copperas, and perhaps in analogy, iron(II) and zinc sulfate were known respectively as green and white copperas.