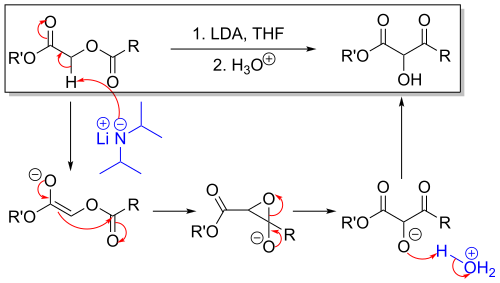
The Chan rearrangement is a chemical reaction that involves rearranging an acyloxy acetate (1) in the presence of a strong base to a 2-hydroxy-3-keto-ester (2).
Contents

This procedure was employed in the Holton Taxol total synthesis.
The Chan rearrangement is a chemical reaction that involves rearranging an acyloxy acetate (1) in the presence of a strong base to a 2-hydroxy-3-keto-ester (2).

This procedure was employed in the Holton Taxol total synthesis.
The methylene bridge in the reactant with adjacent carbonyl and acetyl substituents is acidic and can be deprotonated by strong non-nucleophilic bases such as lithium tetramethylpiperidide or lithium diisopropylamide (LDA) as in an aldol reaction. The thus formed enolate then attacks the adjacent acetyl group through a short lived intermediate oxirane. Acidic workup liberates the free hydroxyl group.