
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group attached to an R-group. The general formula of a carboxylic acid is often written as R−COOH or R−CO2H, sometimes as R−C(O)OH with R referring to an organyl group, or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
The Simmons–Smith reaction is an organic cheletropic reaction involving an organozinc carbenoid that reacts with an alkene to form a cyclopropane. It is named after Howard Ensign Simmons, Jr. and Ronald D. Smith. It uses a methylene free radical intermediate that is delivered to both carbons of the alkene simultaneously, therefore the configuration of the double bond is preserved in the product and the reaction is stereospecific.
Clemmensen reduction is a chemical reaction described as a reduction of ketones or aldehydes to alkanes using zinc amalgam and concentrated hydrochloric acid (HCl). This reaction is named after Erik Christian Clemmensen, a Danish-American chemist.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C3H3N2)2CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:

Nucleophilic conjugate addition is a type of organic reaction. Ordinary nucleophilic additions or 1,2-nucleophilic additions deal mostly with additions to carbonyl compounds. Simple alkene compounds do not show 1,2 reactivity due to lack of polarity, unless the alkene is activated with special substituents. With α,β-unsaturated carbonyl compounds such as cyclohexenone it can be deduced from resonance structures that the β position is an electrophilic site which can react with a nucleophile. The negative charge in these structures is stored as an alkoxide anion. Such a nucleophilic addition is called a nucleophilic conjugate addition or 1,4-nucleophilic addition. The most important active alkenes are the aforementioned conjugated carbonyls and acrylonitriles.

Thiophenol is an organosulfur compound with the formula C6H5SH, sometimes abbreviated as PhSH. This foul-smelling colorless liquid is the simplest aromatic thiol. The chemical structures of thiophenol and its derivatives are analogous to phenols, where the oxygen atom in the hydroxyl group (-OH) bonded to the aromatic ring in phenol is replaced by a sulfur atom. The prefix thio- implies a sulfur-containing compound and when used before a root word name for a compound which would normally contain an oxygen atom, in the case of 'thiol' that the alcohol oxygen atom is replaced by a sulfur atom.
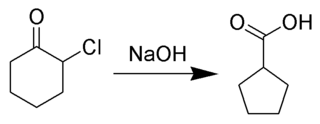
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid, but usually either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds. Note that trihalomethyl ketone substrates will result in haloform and carboxylate formation via the haloform reaction instead.

The Peterson olefination is the chemical reaction of α-silyl carbanions with ketones to form a β-hydroxysilane (2) which eliminates to form alkenes (3).

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc.

Organozinc chemistry is the study of the physical properties, synthesis, and reactions of organozinc compounds, which are organometallic compounds that contain carbon (C) to zinc (Zn) chemical bonds.

Organocadmium chemistry describes the physical properties, synthesis, reactions, and use of organocadmium compounds, which are organometallic compounds containing a carbon to cadmium chemical bond. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited.

In organic chemistry, carbonyl reduction is the conversion of any carbonyl group, usually to an alcohol. It is a common transformation that is practiced in many ways. Ketones, aldehydes, carboxylic acids, esters, amides, and acid halides - some of the most pervasive functional groups, -comprise carbonyl compounds. Carboxylic acids, esters, and acid halides can be reduced to either aldehydes or a step further to primary alcohols, depending on the strength of the reducing agent. Aldehydes and ketones can be reduced respectively to primary and secondary alcohols. In deoxygenation, the alcohol group can be further reduced and removed altogether by replacement with H.
An insertion reaction is a chemical reaction where one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:














