Chemical synthesis is a purposeful execution of chemical reactions to obtain a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions. In modern laboratory usage, this tends to imply that the process is reproducible, reliable, and established to work in multiple laboratories.

In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound.
The following outline is provided as an overview of and topical guide to organic chemistry:

The Grignard reaction is an organometallic chemical reaction in which alkyl, vinyl, or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is important for the formation of carbon–carbon bonds. The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.

An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.

Cinnamic acid is an organic compound with the formula C6H5CH=CHCOOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a cis and a trans isomer, although the latter is more common.
In chemistry, a dehydration reaction is a conversion that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction. Common dehydrating agents used in organic synthesis include sulfuric acid and alumina. Often dehydration reactions are effected with heating.
In chemistry, a trimer is a molecule or an anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization.

N,N'-Dicyclohexylcarbodiimide is an organic compound with the chemical formula (C6H11N)2C. It is a waxy white solid with a sweet odor. It primary use is to couple amino acids during artificial peptide synthesis.The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and dimethylformamide, but insoluble in water. The compound is often abbreviated as DCC, DCCD or DCCI.

Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron transfer in the electrochemical sense of the word. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen

Cyanuric chloride is an organic compound with the formula (NCCl)3. This white solid is the chlorinated derivative of 1,3,5-triazine. It is the trimer of cyanogen chloride. Cyanuric chloride is the main precursor to the popular but controversial herbicide atrazine.
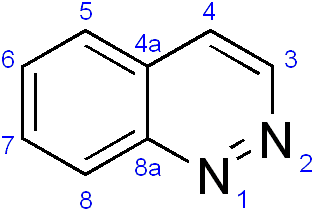
Cinnoline is an aromatic heterocyclic compound with the formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, phthalazine and quinazoline.
1,3,5-triazine, also called s-triazine, is an organic chemical compound with the formula (HCN)3. It is a six-membered heterocyclic aromatic ring, one of several isomeric triazines. S-triazine and its derivatives are useful in a variety of applications.

Cyanuric fluoride or 2,4,6-trifluoro-1,3,5-triazine is a chemical compound with the formula (CNF)3. It is a colourless, pungent liquid. It has been used as a precursor for fibre-reactive dyes, as a specific reagent for tyrosine residues in enzymes, and as a fluorinating agent.

Diphenylphosphoryl azide (DPPA) is an organic compound. It is widely used as a reagent in the synthesis of other organic compounds.
The Boger pyridine synthesis is a cycloaddition approach to the formation of pyridines named after its inventor Dale L. Boger, who first reported it in 1981. The reaction is a form of inverse-electron demand Diels-Alder reaction in which an enamine reacts with a 1,2,4-triazine to form the pyridine nucleus. The reaction is especially useful for accessing pyridines that would be difficult or impossible to access via other methods and has been used in the total synthesis of several complicated natural products.

DMTMM is an organic triazine derivative commonly used for activation of carboxylic acids, particularly for amide synthesis. Amide coupling is one of the most common reactions in organic chemistry and DMTMM is one reagent used for that reaction. The mechanism of DMTMM coupling is similar to other common amide coupling reactions involving activated carboxylic acids. Its precursor, 2-chloro-4,6,-dimethoxy-1,3,5-triazine (CDMT), has also been used for amide coupling. DMTMM has also been used to synthesize other carboxylic functional groups such as esters and anhydrides. DMTMM is usually used in the chloride form but the tetrafluoroborate salt is also commercially available.
















