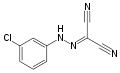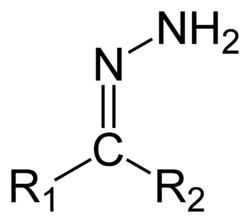
Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. [1] They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. [2] [3]