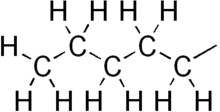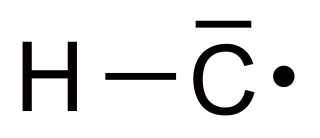Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It was first documented in 1844 and came into medical use in 1867.

Methyl radical is an organic compound with the chemical formula CH•
3. It is a metastable colourless gas, which is mainly produced in situ as a precursor to other hydrocarbons in the petroleum cracking industry. It can act as either a strong oxidant or a strong reductant, and is quite corrosive to metals.
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of −CnH2n+1. A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula −CnH2n−1. Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula −CH3.

Iodoform is the organoiodine compound with the chemical formula CHI3. It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.

Amyl nitrate is the chemical compound with the formula CH3(CH2)4ONO2. This molecule consists of the 5-carbon amyl group attached to a nitrate functional group. It is the ester of amyl alcohol and nitric acid.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
In organic chemistry, a propyl group is a three-carbon alkyl substituent with chemical formula −CH2CH2CH3 for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr.

Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.
In organic chemistry, a substituent is one or a group of atoms that replaces atoms, thereby becoming a moiety in the resultant (new) molecule.

Amyl acetate (pentyl acetate) is an organic compound and an ester with the chemical formula CH3COO[CH2]4CH3 and the molecular weight 130.19 g/mol. It is colorless and has a scent similar to bananas and apples. The compound is the condensation product of acetic acid and 1-pentanol. However, esters formed from other pentanol isomers (amyl alcohols), or mixtures of pentanols, are often referred to as amyl acetate. The symptoms of exposure to amyl acetate in humans are dermatitis, central nervous system depression, narcosis and irritation to the eyes and nose.
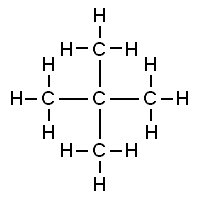
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.
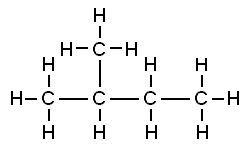
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).

Phthalazine, also called benzo-orthodiazine or benzopyridazine, is a heterocyclic organic compound with the molecular formula C8H6N2. It is isomeric with other naphthyridines including quinoxaline, cinnoline and quinazoline.
2-Chloroethanol (also called ethylene chlorohydrin or glycol chlorohydrin) is an organic chemical compound with the chemical formula HOCH2CH2Cl and the simplest beta-halohydrin (chlorohydrin). This colorless liquid has a pleasant ether-like odor. It is miscible with water. The molecule is bifunctional, consisting of both an alkyl chloride and an alcohol functional group.

Isoamyl alcohol is a colorless liquid with the formula C
5H
12O, specifically (H3C–)2CH–CH2–CH2–OH. It is one of several isomers of amyl alcohol (pentanol). It is also known as isopentyl alcohol, isopentanol, or (in the IUPAC recommended nomenclature) 3-methyl-butan-1-ol. An obsolete name for it was isobutyl carbinol.

Benzopyran is a polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring.
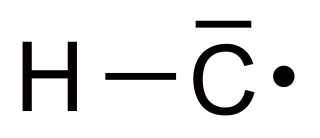
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other functional groups for the hydrogen.

Phenyl isothiocyanate (PITC) is a reagent used in reversed phase HPLC. PITC is less sensitive than o-phthaldehyde (OPA) and cannot be fully automated. PITC can be used for analysing secondary amines, unlike OPA. It is also known as Edman's reagent and is used in Edman degradation.
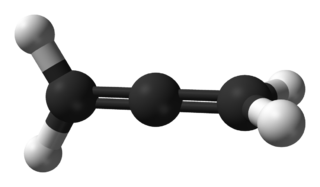
Propadiene or allene is the organic compound with the formula H2C=C=CH2. It is the simplest allene, i.e. a compound with two adjacent carbon double bonds. As a constituent of MAPP gas, it has been used as a fuel for specialized welding.