
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

Sodium percarbonate, or sodium carbonate peroxide is a chemical substance with formula Na
2H
3CO
6. It is an adduct of sodium carbonate and hydrogen peroxide whose formula is more properly written as 2 Na
2CO
3 · 3 H
2O
2. It is a colorless, crystalline, hygroscopic and water-soluble solid. It is sometimes abbreviated as SPC. It contains 32.5% by weight of hydrogen peroxide.

Acetone peroxide is an organic peroxide and a primary explosive. It is produced by the reaction of acetone and hydrogen peroxide to yield a mixture of linear monomer and cyclic dimer, trimer, and tetramer forms. The dimer is known as diacetone diperoxide (DADP). The trimer is known as triacetone triperoxide (TATP) or tri-cyclic acetone peroxide (TCAP). Acetone peroxide takes the form of a white crystalline powder with a distinctive bleach-like odor or a fruit-like smell when pure, and can explode powerfully if subjected to heat, friction, static electricity, concentrated sulfuric acid, strong UV radiation or shock. Until about 2015, explosives detectors were not set to detect non-nitrogenous explosives, as most explosives used preceding 2015 were nitrogen-based. TATP, being nitrogen-free, has been used as the explosive of choice in several terrorist bomb attacks since 2001.

Hydrogen peroxide - urea is a white crystalline solid chemical compound composed of equal amounts of hydrogen peroxide and urea. It contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as an oxidizing agent. Often called carbamide peroxide in dentistry, it is used as a source of hydrogen peroxide when dissolved in water for bleaching, disinfection and oxidation.
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid. It is a white solid often used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.

Perrhenic acid is the chemical compound with the formula Re2O7(H2O)2. It is obtained by evaporating aqueous solutions of Re2O7. Conventionally, perrhenic acid is considered to have the formula HReO4, and a species of this formula forms when rhenium(VII) oxide sublimes in the presence of water or steam. When a solution of Re2O7 is kept for a period of months, it breaks down and crystals of HReO4·H2O are formed, which contain tetrahedral ReO−4. For most purposes, perrhenic acid and rhenium(VII) oxide are used interchangeably. Rhenium can be dissolved in nitric or concentrated sulfuric acid to produce perrhenic acid.
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as O2Se(OH)2. It is a colorless compound. Although it has few uses, one of its salts, sodium selenate is used in the production of glass and animal feeds.
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments.

Hydroperoxides or peroxols are compounds of the form ROOH, where R stands for any group, typically organic, which contain the hydroperoxy functional group. Hydroperoxide also refers to the hydroperoxide anion and its salts, and the neutral hydroperoxyl radical (•OOH) consist of an unbond hydroperoxy group. When R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with unsaturated chemical bonds.
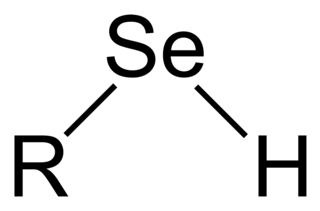
Selenols are organic compounds that contain the functional group with the connectivity C−Se−H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. A well-known selenol is the amino acid selenocysteine.

The Dakin oxidation (or Dakin reaction) is an organic redox reaction in which an ortho- or para-hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone reacts with hydrogen peroxide (H2O2) in base to form a benzenediol and a carboxylate. Overall, the carbonyl group is oxidised, whereas the H2O2 is reduced.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO2H and RSeO3H, respectively. Selenenic acids derived from selenoenzymes are thought to be responsible for the antioxidant activity of these enzymes. This functional group is sometimes called SeO-selenoperoxol.

Methaneseleninic acid is an organoselenium compound, a seleninic acid with the chemical formula CH3SeO2H. Its structure is CH3−Se(=O)−OH.

In organic chemistry, alkenyl peroxides are organic compounds bearing an alkene residue directly at the peroxide group, resulting in the general formula R2C=C(R)OOR. They have very weak O-O bonds and are thus generally unstable compounds.

Trifluoroperacetic acid is an organofluorine compound, the peroxy acid analog of trifluoroacetic acid, with the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selones. It is a potentially explosive material and is not commercially available, but it can be quickly prepared as needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.

Bis(trimethylsilyl)peroxide (sometimes abbreviated as BTSP) is an organosilicon compound with the formula ((CH3)3SiO)2. It is a colorless liquid that is soluble in organic solvents so long as they lack acidic groups. The compound represents an aprotic analogue of hydrogen peroxide and as such it is used for certain sensitive organic oxidations. Upon treatment with organolithium compounds, it affords the silyl ether.

Peroxymonophosphoric acid is an oxyacid of phosphorus. It is a colorless viscous oil. Its salts are called peroxymonophosphates. Another peroxyphosphoric acid is peroxydiphosphoric acid, H4P2O8.

In chemistry, a selenosulfide refers to distinct classes of inorganic and organic compounds containing sulfur and selenium. The organic derivatives contain Se-S bonds, whereas the inorganic derivatives are more variable.















