This article needs additional citations for verification .(August 2022) |

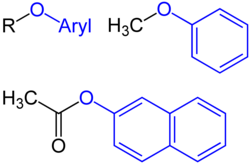
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus R−O. Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (CH3O−). [1] An ethoxy group (CH3CH2O−) is found in the organic compound ethyl phenyl ether (C6H5OCH2CH3, also known as ethoxybenzene).
Related to alkoxy groups are aryloxy groups, which have an aryl group singularly bonded to oxygen such as the phenoxy group (C6H5O−).
An alkoxy or aryloxy group bonded to an alkyl or aryl (R−O−R') is an ether. If bonded to H it is an alcohol.
The term alkoxide refers to the anionic conjugate bases of alcohols (RO−) or to ionic compounds containing such an anion. Alkoxide compounds are derivatives of alcohols where the hydrogen of the –OH group is replaced by a metal; [2] for example, the sodium salt of ethanol (CH3CH2OH) is sodium ethoxide, containing ethoxide anions CH3CH2O− and sodium cations Na+.