An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Ammonium perchlorate ("AP") is an inorganic compound with the formula NH4ClO4. It is a colorless or white solid that is soluble in water. It is a powerful oxidizer. Combined with a fuel, it can be used as a rocket propellant called ammonium perchlorate composite propellant. Its instability has involved it in a number of accidents, such as the PEPCON disaster.

Perchloric acid is a mineral acid with the formula HClO4. It is an oxoacid of chlorine. Usually found as an aqueous solution, this colorless compound is a stronger acid than sulfuric acid, nitric acid and hydrochloric acid. It is a powerful oxidizer when hot, but aqueous solutions up to approximately 70% by weight at room temperature are generally safe, only showing strong acid features and no oxidizing properties. Perchloric acid is useful for preparing perchlorate salts, especially ammonium perchlorate, an important rocket fuel component. Perchloric acid is dangerously corrosive and readily forms potentially explosive mixtures.

Hydrazine is an inorganic compound with the chemical formula N2H4. It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate.
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH
3NO
2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.

Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.

Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
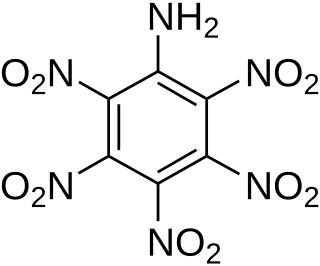
Pentanitroaniline, sometimes called hexyl, is an explosive organic compound. It is a relatively sensitive explosive that can be used as a base charge for detonators, although it is uncommon in this application.

Azines are a functional class of organic compounds with the connectivity RR'C=N-N=CRR'. These compounds are the product of the condensation of hydrazine with ketones and aldehydes, although in practice they are often made by alternative routes. Ketazines are azines derived from ketones. For example, acetone azine is the simplest ketazine. Aldazines are azines derived from aldehydes.

Semicarbazide is the chemical compound with the formula OC(NH2)(N2H3). It is a water-soluble white solid. It is a derivative of urea.
The chemical element nitrogen is one of the most abundant elements in the universe and can form many compounds. It can take several oxidation states; but the most common oxidation states are -3 and +3. Nitrogen can form nitride and nitrate ions. It also forms a part of nitric acid and nitrate salts. Nitrogen compounds also have an important role in organic chemistry, as nitrogen is part of proteins, amino acids and adenosine triphosphate.
A pyrotechnic composition is a substance or mixture of substances designed to produce an effect by heat, light, sound, gas/smoke or a combination of these, as a result of non-detonative self-sustaining exothermic chemical reactions. Pyrotechnic substances do not rely on oxygen from external sources to sustain the reaction.
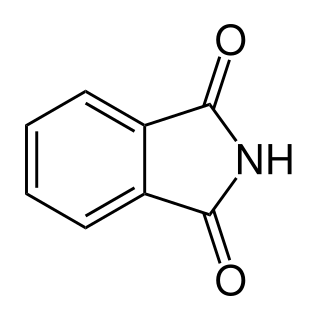
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor to other organic compounds as a masked source of ammonia.

Xylitol pentanitrate (XPN) is a nitrated ester primary explosive first synthesized in 1891 by Gabriel Bertrand. Law enforcement has taken an interest in XPN along with erythritol tetranitrate (ETN) and pentaerythritol tetranitrate (PETN) due to their ease of synthesis, which makes them accessible to amateur chemists and terrorists.

Ammonium dinitramide (ADN) is an inorganic compound with the chemical formula [NH4][N(NO2)2]. It is the ammonium salt of dinitraminic acid HN(NO2)2. It consists of ammonium cations [NH4]+ and dinitramide anions −N(NO2)2. ADN decomposes under heat to leave only nitrogen, oxygen, and water.

In chemistry, the pentazenium cation is a positively-charged polyatomic ion with the chemical formula N+5 and structure N−N−N−N−N. Together with solid nitrogen polymers and the azide anion, it is one of only three poly-nitrogen species obtained in bulk quantities.
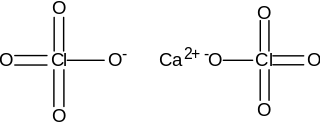
Calcium perchlorate is classified as a metal perchlorate salt with the molecular formula Ca(ClO4)2. It is an inorganic compound that is a yellow-white crystalline solid in appearance. As a strong oxidizing agent, it reacts with reducing agents when heated to generate heat and products that may be gaseous. Calcium perchlorate has been categorized as having explosive reactivity. Ca(ClO4)2 is a common chemical on the soil of planet Mars, counting for almost 1% of the Martian dust, by weight.

Hydrazinium is the cation with the formula [N2H5]+. This cation has a methylamine-like structure. It can be derived from hydrazine by protonation. Hydrazinium is a weak acid with pKa = 8.1.

Nickel hydrazine nitrate (NHN), (chemical formula: [Ni(N2H4)3](NO3)2 is an energetic material having explosive properties in between that of primary explosive and a secondary explosive. It is a salt of a coordination compound of nickel with a reaction equation of 3N2H4·H2O + Ni(NO3)2 →〔Ni(N2H4)3〕(NO3)2 + 3H2O
Azidotetrazolate (CN7−) is an anion which forms a highly explosive series of salts. The ion is made by removing a proton from 5-azido-1H-tetrazole. The molecular structure contains a five-membered ring with four nitrogen atoms, and an azido side chain connected to the carbon atom. Several salts exist, but they are unstable and spontaneously explode. For example, the rubidium, potassium and caesium salts are so unstable that they explode while crystallizing.