Alcohol dehydrogenases (ADH) (EC 1.1.1.1) are a group of dehydrogenase enzymes that occur in many organisms and facilitate the interconversion between alcohols and aldehydes or ketones with the reduction of nicotinamide adenine dinucleotide (NAD+) to NADH. In humans and many other animals, they serve to break down alcohols that are otherwise toxic, and they also participate in the generation of useful aldehyde, ketone, or alcohol groups during the biosynthesis of various metabolites. In yeast, plants, and many bacteria, some alcohol dehydrogenases catalyze the opposite reaction as part of fermentation to ensure a constant supply of NAD+.
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light and air because it forms peroxides.

Adipic acid or hexanedioic acid is the organic compound with the formula (CH2)4(COOH)2. From an industrial perspective, it is the most important dicarboxylic acid: about 2.5 billion kilograms of this white crystalline powder are produced annually, mainly as a precursor for the production of nylon. Adipic acid otherwise rarely occurs in nature, but it is known as manufactured E number food additive E355. Salts and esters of adipic acid are known as adipates.

Mucic acid, C6H10O8 or HOOC-(CHOH)4-COOH (galactaric acid or meso-galactaric acid) is an aldaric acid obtained by nitric acid oxidation of galactose or galactose-containing compounds such as lactose, dulcite, quercite, and most varieties of gum.

Isoquinoline is a heterocyclic aromatic organic compound. It is a structural isomer of quinoline. Isoquinoline and quinoline are benzopyridines, which are composed of a benzene ring fused to a pyridine ring. In a broader sense, the term isoquinoline is used to make reference to isoquinoline derivatives. 1-Benzylisoquinoline is the structural backbone in naturally occurring alkaloids including papaverine. The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
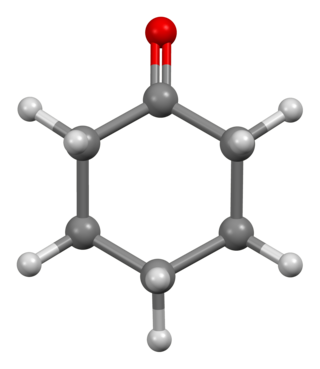
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexanone assume a pale yellow color. Cyclohexanone is slightly soluble in water and miscible with common organic solvents. Billions of kilograms are produced annually, mainly as a precursor to nylon.

Hair spray is a common cosmetic hairstyling product that is sprayed onto hair to protect against humidity and wind and have it stay in a desired shape. Hair sprays typically consist of several components for the hair as well as a propellant.

A toxoid is an inactivated toxin whose toxicity has been suppressed either by chemical (formalin) or heat treatment, while other properties, typically immunogenicity, are maintained. Toxins are secreted by bacteria, whereas toxoids are altered form of toxins; toxoids are not secreted by bacteria. Thus, when used during vaccination, an immune response is mounted and immunological memory is formed against the molecular markers of the toxoid without resulting in toxin-induced illness. Such a preparation is also known as an anatoxin. There are toxoids for prevention of diphtheria, tetanus and botulism.

Soured milk denotes a range of food products produced by the acidification of milk. Acidification, which gives the milk a tart taste, is achieved either through bacterial fermentation or through the addition of an acid, such as lemon juice or vinegar. The acid causes milk to coagulate and thicken, inhibiting the growth of harmful bacteria and improving the product's shelf life.

Glutaric acid is the organic compound with the formula C3H6(COOH)2. Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50% (w/w).

Pimelic acid is the organic compound with the formula HO2C(CH2)5CO2H. Pimelic acid is one CH
2 unit longer than a related dicarboxylic acid, adipic acid, a precursor to many polyesters and polyamides. However compared to adipic acid, pimelic acid is relatively small in importance industrially. Derivatives of pimelic acid are involved in the biosynthesis of the amino acid lysine and the vitamin biotin.
Dioctyl adipate (DOA) is an organic compound with the formula (CH2CH2CO2C8H17)2. It is a colorless oily liquid. As well as related diesters derived from 2-ethylhexanol, decanol, isodecanol, etc., it is used as a plasticizer.

Glycol nucleic acid (GNA), sometimes also referred to as glycerol nucleic acid, is a nucleic acid similar to DNA or RNA but differing in the composition of its sugar-phosphodiester backbone, using propylene glycol in place of ribose or deoxyribose. GNA is chemically stable but not known to occur naturally. However, due to its simplicity, it might have played a role in the evolution of life.
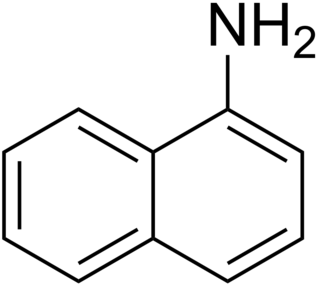
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer. It crystallizes in colorless needles which melt at 50 °C. It possesses a disagreeable odor, sublimes readily, and turns brown on exposure to air. It is the precursor to a variety of dyes.
PBAT is a biodegradable random copolymer, specifically a copolyester of adipic acid, 1,4-butanediol and terephthalic acid. PBAT is produced by many different manufacturers and may be known by the brand names ecoflex, Wango,Ecoworld, Eastar Bio, and Origo-Bi. It is also called poly(butylene adipate-co-terephthalate) and sometimes polybutyrate-adipate-terephthalate or even just "polybutyrate". It is generally marketed as a fully biodegradable alternative to low-density polyethylene, having many similar properties including flexibility and resilience, allowing it to be used for many similar uses such as plastic bags and wraps. It is depicted as a block co-polymer here due to the common synthetic method of first synthesizing two copolymer blocks and then combining them. However, it is important to note that the actual structure of the polymer is a random co-polymer of the blocks shown.
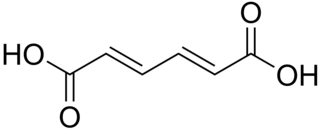
Muconic acid is a dicarboxylic acid. There are three isomeric forms designated trans,trans-muconic acid, cis,trans-muconic acid, and cis,cis-muconic acid which differ by the geometry around the double bonds. Its name is derived from mucic acid.
The molecular formula C6H14N4O2 (molar mass: 174.20 g/mol, exact mass: 174.1117 u) may refer to:
Bis(2-ethylhexyl) adipate or DEHA or DOA is an organic compound with the formula (CH2CH2CO2C8H17)2. It is the diester of 2-ethylhexanol and adipic acid. It is a colorless oily liquid.

Nitrolic acids are organic compounds with the functional group RC(NO2)=NOH. They are prepared by the reaction of nitroalkanes with base and nitrite sources:














